Search found 165 matches
- Sun Mar 17, 2019 9:48 am
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: 6M5 a) 7th Edition
- Replies: 1
- Views: 419
Re: 6M5 a) 7th Edition
NO3- is being reduced, not oxidized, which is why it is at the cathode. N is being reduced from a +5 oxidation state in NO3- to a +2 oxidation state in NO. Hg is being oxidized from an oxidation state of 0 to an oxidation state of +1 in Hg2 2+
- Sun Mar 17, 2019 9:44 am
- Forum: Reaction Mechanisms, Reaction Profiles
- Topic: Reaction Profiles
- Replies: 3
- Views: 670
Re: Reaction Profiles
The peaks are not exactly based on the intermediates; they are based on the activation energy for the step in the mechanism. If a step is slow, there will be a greater distance from the reactant/intermediate to the peak (higher Ea), whereas if a step is fast, there will be a smaller distance (low Ea).
- Sun Mar 17, 2019 9:31 am
- Forum: General Science Questions
- Topic: Midterm Q3D
- Replies: 1
- Views: 535
Re: Midterm Q3D
H3PO4 is a polyprotic acid, so it will have three deprotonations where it gives up H+. Ka2 is the acidity constant for the second deprotonation, so you have to use H2PO4- (it lost H+ in the first deprotonation, it may be helpful to write this reaction out too) and have it react with water, donating ...
- Sun Mar 17, 2019 9:21 am
- Forum: General Rate Laws
- Topic: fast step before slow step
- Replies: 2
- Views: 592
Re: fast step before slow step
This is not a general assumption. It's most likely that we will be given whether a step is slow or fast. Fast step before the slow step is a condition that must be met in order to do the pre-equilbrium approach. If the slow step is the first step, then it is the rate determining step and its rate la...
- Sun Mar 17, 2019 9:19 am
- Forum: Balancing Redox Reactions
- Topic: How to balance half reaction
- Replies: 5
- Views: 836
Re: How to balance half reaction
The way I like to do it is to balance the reaction the same way I would if it were acidic, then adding OH- to both sides to neutralize the H+. When you do that, H+ and OH- combine to make H2O, so you've essentially only added H2O and OH-.
- Sun Mar 17, 2019 9:17 am
- Forum: Kinetics vs. Thermodynamics Controlling a Reaction
- Topic: intermediate
- Replies: 26
- Views: 2075
Re: intermediate
An intermediate is a species that is formed and subsequently used up in a reaction mechanism. It can't appear in the rate law because it is not part of the overall reaction.
- Sun Mar 17, 2019 9:14 am
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Platinum Electrode
- Replies: 3
- Views: 530
Re: Platinum Electrode
You need a solid platinum electrode when there is no conducting solid (except liquid mercury) in the cathode or anode reaction. That's all you need to know.
- Sun Mar 17, 2019 9:12 am
- Forum: First Order Reactions
- Topic: 1st order decay?
- Replies: 2
- Views: 494
Re: 1st order decay?
Yes. If you raise e to the power of both sides of the integrated rate law, you will get 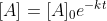 , which is exponential decay. Keep in mind that this is with respect to reactant.
, which is exponential decay. Keep in mind that this is with respect to reactant.
- Sun Mar 17, 2019 9:10 am
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Test #2: Question #4, Parts A and B
- Replies: 2
- Views: 571
Re: Test #2: Question #4, Parts A and B
I have a slightly different version of the test so I can't give you the exact answers, but where you went wrong was that you made a cell that had a negative cell potential. If you are asked to construct a galvanic cell, you have to make sure that the cell potential is positive so that it can do usef...
- Sun Mar 17, 2019 9:04 am
- Forum: Reaction Mechanisms, Reaction Profiles
- Topic: Kinetic Plot Graphs
- Replies: 1
- Views: 485
Re: Kinetic Plot Graphs
You can obtain this relationship from the differential rate law: -\frac{1}{a}\frac{d[A]}{dt}=k[A] . If you think about it in terms of y=mx+b, you will see that y is d[A]/dt, the slope is -ka, and x is [A]. With the same reasoning, you can use the equation for half-life for a zero-order reaction to s...
- Sun Mar 17, 2019 8:54 am
- Forum: Work, Gibbs Free Energy, Cell (Redox) Potentials
- Topic: Difference between ΔG and ∆Go
- Replies: 1
- Views: 443
Re: Difference between ΔG and ∆Go
The difference between delta G and delta G naught is that delta G naught is the change in free energy under standard conditions. The change in free energy is the maximum work, so both equations are correct, but it just depends on the conditions.
- Sun Mar 17, 2019 8:50 am
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: OH- in cell diagram
- Replies: 1
- Views: 428
Re: OH- in cell diagram
You should include OH- in your cell diagram to indicate that the reaction is occurring in basic conditions. You don't need to include water because it's implied when you say that the solution is aqueous.
- Sat Mar 16, 2019 2:33 pm
- Forum: Reaction Mechanisms, Reaction Profiles
- Topic: Reaction Mechanisms without unknown rate-determining step
- Replies: 2
- Views: 325
Re: Reaction Mechanisms without unknown rate-determining step
It's very unlikely that we would have to figure this out for ourselves as we've always been given which steps are fast and which step is slow. I suppose one way you could think about which step is slow is by looking at the molecularity. If the molecularity is high, we would expect the rate constant ...
- Wed Mar 13, 2019 10:21 pm
- Forum: First Order Reactions
- Topic: Integrated Rate Law
- Replies: 2
- Views: 336
Re: Integrated Rate Law
It doesn't matter since they are just different versions of each other and you will get the same answer either way. It really just depends on what you're solving for (one version may be easier to use) or personal preference.
- Wed Mar 13, 2019 10:18 pm
- Forum: Arrhenius Equation, Activation Energies, Catalysts
- Topic: subtracting .59? 7D.5 7th ed
- Replies: 1
- Views: 254
Re: subtracting .59? 7D.5 7th ed
It's a typo. The minus sign should be an equals sign.
- Wed Mar 13, 2019 10:16 pm
- Forum: General Rate Laws
- Topic: Molarity and rates
- Replies: 1
- Views: 231
Re: Molarity and rates
I think you're approaching this in an slightly incorrect manner. Remember that rate is dependent on both the rate constant and the concentrations of the reactants. If the concentrations of the reactants are high, the probability of a successful collision (and therefore a reaction) is not changing; t...
- Wed Mar 13, 2019 10:05 pm
- Forum: Arrhenius Equation, Activation Energies, Catalysts
- Topic: Determining Catalysts
- Replies: 2
- Views: 328
Re: Determining Catalysts
In general, yes, catalysts are there to begin with, whereas intermediates are formed during the reaction mechanism. Both are neither reactants nor products. I think that it is possible to have catalysts in different steps though, since a couple HW questions asked about the effect of adding catalysts...
- Sun Mar 10, 2019 7:11 pm
- Forum: Second Order Reactions
- Topic: 7B.13 Help
- Replies: 4
- Views: 481
Re: 7B.13 Help
Since the half-life of a second-order reaction depends of [A]0, you can't just multiply the initial half-life by the number of half-lives because you have a different [A]0 for each half-life. Therefore, you have to find k using the equation t_{1/2}=\frac{1}{k[A]_{0}} Once you have k, you can plug in...
- Sun Mar 10, 2019 7:04 pm
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: 7th edition 6.79
- Replies: 1
- Views: 253
Re: 7th edition 6.79
This problem was brought up to Dr. Lavelle during office hours and he said to omit that problem since we never covered current.
- Sun Mar 10, 2019 7:03 pm
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: 7th edition 6.57
- Replies: 1
- Views: 329
Re: 7th edition 6.57
They take the square root because K is for the reaction 2HF --> 2H+ + 2F-, but Ka is for the reaction HF <--> H+ + F-. Since the reaction for Ka is half of that of K, Ka is the square root of K.
- Sun Mar 10, 2019 6:48 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: identifying cathode anode
- Replies: 4
- Views: 667
Re: identifying cathode anode
If you want the cell to be able to do useful work (have a positive cell potential), then yes, you want the more positive reduction potential at the cathode. However, there are cases such as the problem mentioned in this thread where you are given a reaction and you have to go off of that in terms of...
- Tue Mar 05, 2019 11:55 pm
- Forum: General Rate Laws
- Topic: Rate Laws
- Replies: 1
- Views: 212
Re: Rate Laws
If the reactant does not affect the rate of the reaction, then the rate is zero-order in that reactant. Therefore the coefficient in the general rate law is 0, and anything to the 0 power is 1, so you can eliminate it from the rate law equation.
- Mon Mar 04, 2019 12:25 am
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: Low voltage in concentration cells
- Replies: 2
- Views: 361
Re: Low voltage in concentration cells
Conceptually, E naught is 0 because at standard conditions, the solutions at the anode and cathode are both 1M. If you have a concentration cell, the solutions will contain the same ion, and if they are both 1M, then they will be in equilibrium. A reaction at equilibrium has a deltaG of 0, so E is 0...
- Mon Mar 04, 2019 12:17 am
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: 6th edition 14.101
- Replies: 1
- Views: 944
Re: 6th edition 14.101
You can treat this problem as you would a concentration cell. The potential difference is caused by a concentration difference/gradient of K+. Since you're dealing with a "concentration cell", E naught is 0. The reduction of K+ involves only one electron, so n is 1. To find Q, remember tha...
- Mon Mar 04, 2019 12:10 am
- Forum: Work, Gibbs Free Energy, Cell (Redox) Potentials
- Topic: Endergonic vs. Endothermic
- Replies: 6
- Views: 1933
Re: Endergonic vs. Endothermic
These two terms refer to different thermodynamic state functions. An endergonic reaction is one that has a positive change in Gibb's free energy (non-spontaneous). An endothermic reaction is one that has a positive change in enthalpy (requires heat).
- Mon Mar 04, 2019 12:07 am
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: 6N.15 7th Ed
- Replies: 1
- Views: 307
Re: 6N.15 7th Ed
If the pH is 11, you know that the solution is basic. You would get that by adding OH- to solution via NaOH. However, in Table 6I.1, Ni(OH)2 has a Ksp of 6.5x10^-18, which means that Ni(OH)2 (s) is heavily favored vs. Ni2+ and OH- ions in solution. Therefore, in the context of this problem, when you...
- Wed Feb 27, 2019 11:11 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Redox Reactions and Cell Potentials
- Replies: 2
- Views: 296
Re: Redox Reactions and Cell Potentials
As Dr. Lavelle emphasized during lecture, standard reduction potential is an intensive property. This means that it does not depend on the amount of times the reaction occurs.
- Wed Feb 27, 2019 11:09 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: HW problem 5G.17 (7th edition)
- Replies: 1
- Views: 200
Re: HW problem 5G.17 (7th edition)
In 5G.13, you calculated deltaG for the reaction: I2(g) --> 2I(g). You had the correct answer in that deltaG was positive so reactants (I2) were favored. In 5G.17, they reversed the reaction because that is the spontaneous reaction, but the answer is still correct as I2 forms ([I2] increases while [...
- Wed Feb 27, 2019 11:00 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: 6M.5 part c 7th edition
- Replies: 2
- Views: 163
Re: 6M.5 part c 7th edition
I think that you assigned your oxidation numbers incorrectly. Hg(l) has an oxidation state of 0 while Hg in Hg2 2+ has an oxidation state of +1. Since Hg is oxidized, this half reaction takes place at the anode. N in NO3- has an oxidation state of +5 (-1 = 3*(-2) + 5) while N in NO has an oxidation ...
- Wed Feb 27, 2019 10:56 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: identifying cathode anode
- Replies: 4
- Views: 667
Re: identifying cathode anode
It is true that we want the cathode to have a greater positive reduction potential so that the galvanic cell will be able to do useful work (positive cell potential). However, the question never specified that the cell had to have a positive cell potential. It merely asks you to calculate the standa...
- Sun Feb 24, 2019 11:57 pm
- Forum: Work, Gibbs Free Energy, Cell (Redox) Potentials
- Topic: 6M7a 7th Ed
- Replies: 1
- Views: 135
Re: 6M7a 7th Ed
You can't use the periodic table because transition metals don't exactly follow electronegativity trends. For this problem, you would have to look at the standard reduction potentials in Appendix 2B. The more positive the reduction potential, the weaker reducing agent it is.
- Sun Feb 24, 2019 11:48 pm
- Forum: Work, Gibbs Free Energy, Cell (Redox) Potentials
- Topic: determining n
- Replies: 4
- Views: 524
Re: determining n
In the equation deltaG = -nFE, n refers to the moles of electrons transferred. You can typically find this by breaking up an already balanced equation into its respective oxidation and reduction half-reactions or by balancing two known oxidation and reduction half-reactions. For example, in the Zn-C...
- Sun Feb 24, 2019 12:36 am
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: 7th Edit 6L5 b
- Replies: 2
- Views: 314
Re: 7th Edit 6L5 b
To further clarify, you need Pt(s) as it acts as an inert electrode. It doesn't participate directly in the reaction but it is necessary to for electron transfer to occur.
- Sun Feb 24, 2019 12:32 am
- Forum: Balancing Redox Reactions
- Topic: 14.5d
- Replies: 2
- Views: 237
Re: 14.5d
Your final equation is not balanced; you have 12 H on the left side (6*2), but 18 on the right side (4*3+6). In addition, your charges aren't balanced either; the left side has a -12 charge (12e-) and the right side has a -6 charge (6OH-)
- Sun Feb 24, 2019 12:24 am
- Forum: Balancing Redox Reactions
- Topic: Rules for oxidation numbers
- Replies: 2
- Views: 424
Re: Rules for oxidation numbers
I would memorize the following: 1. Any element by itself (no charge) has an oxidation number of 0 (H2, Cl2, O2, O3, etc.) 2. H typically has an oxidation number of +1 3. O typically as an oxidation number of -2 (except in H2O2 where it's -1) 4. Halogens (Cl, F, Br, I) typically have an oxidation num...
- Sun Feb 24, 2019 12:16 am
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: 6L.11 7th Edition
- Replies: 1
- Views: 264
Re: 6L.11 7th Edition
I'm assuming you meant 6L.9. If you look in Appendix 2B, there is only one reduction half-reaction containing Fe2+ that you can reverse into an oxidation half-reaction where Fe2+ is oxidized. Of course, you would have to deduce this after you found that MnO4- is reduced, again by looking at Appendix...
- Fri Feb 22, 2019 11:39 pm
- Forum: Balancing Redox Reactions
- Topic: Homework 14.5 part d
- Replies: 4
- Views: 462
Re: Homework 14.5 part d
For letter d) in problem 14.5 (6th edition), the answe key says to multiply the first half-reaction by 3, but then the new reaction contains 4P4, not 3P4. Is this a mistake in the answer key or the actual answer? If it’s correct, could someone explain why? The overall redox reaction has 4P4 because...
- Fri Feb 22, 2019 11:33 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: HW problem 5.39 7th edition
- Replies: 1
- Views: 232
Re: HW problem 5.39 7th edition
You are correct in using the ICE table; however, you should not make Kc negative. That doesn't make sense as Kc is a ratio of products to reactants, and it's impossible to have a negative concentration. If you recall, when you change the reaction (multiplying by a factor, reversing the reaction), yo...
- Wed Feb 20, 2019 11:28 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Porous disc explanation?
- Replies: 6
- Views: 638
Re: Porous disc explanation?
A quick correction to some of the answers: a salt bridge or porous disc allows ion transfer, not electron transfer. Electron transfer takes place along the wire (hence the electric current). In the Cu/Zn galvanic cell example, the salt bridge provides anions to the anode beaker because Zn is being o...
- Wed Feb 20, 2019 11:22 pm
- Forum: Entropy Changes Due to Changes in Volume and Temperature
- Topic: Midterm Question
- Replies: 8
- Views: 792
Re: Midterm Question
It was also important to note that nitrogen is a diatomic gas. The values on the equation sheet are those for monatomic ideal gases, so you would have to add R to each for diatomic ideal gases (therefore Cv,m for N2 is about (5/2)R). You had to use Cv,m because you broke it up into two steps: increa...
- Wed Feb 20, 2019 11:16 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: 6th edition 9.67
- Replies: 3
- Views: 409
Re: 6th edition 9.67
The way I like to do this is to set up an inequality from the beginning: deltaG < 0 because we want the temperatures for which the reaction is spontaneous. This makes it easy to see what range of temperatures for which the reaction is spontaneous. Sometimes you will have a range of temperature value...
- Mon Feb 11, 2019 10:47 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: No Change in Volume
- Replies: 1
- Views: 211
Re: No Change in Volume
I don't recall any situations in lecture or homework that involve this situation since a change in moles of gas is always accompanied by a change in volume. You use delta nRT as a substitution for PdeltaV when you know the change in moles of gas but don't know the change in volume.
- Mon Feb 11, 2019 10:43 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: 2 molar heat capacities of ideal gas
- Replies: 1
- Views: 258
Re: 2 molar heat capacities of ideal gas
In simple terms, at constant volume, adding heat to the gas would only raise its temperature, but at constant pressure, some of the energy supplied as heat also goes towards expansion work. Thus, it would take a greater amount of heat to raise the temperature of an ideal gas by 1 degree Celsius unde...
- Mon Feb 11, 2019 6:37 pm
- Forum: Third Law of Thermodynamics (For a Unique Ground State (W=1): S -> 0 as T -> 0) and Calculations Using Boltzmann Equation for Entropy
- Topic: 1 molecule
- Replies: 2
- Views: 267
Re: 1 molecule
1 mole is 6.022 x 10^23 molecules, so when they give you 1 molecule it's just 1 molecule.
- Fri Feb 08, 2019 11:44 pm
- Forum: Entropy Changes Due to Changes in Volume and Temperature
- Topic: 9.13 6th Edition
- Replies: 1
- Views: 200
Re: 9.13 6th Edition
This is a error in the solutions manual; if you check the website under "Solution Manual Errors 6th Edition" it is there. The equation in the textbook and on the equations and constants sheet is \Delta S=nCln\frac{T_{2}}{T_{1}} and should be the one you use. The corresponding problem in th...
- Wed Feb 06, 2019 10:34 pm
- Forum: Calculating Work of Expansion
- Topic: 6th edition 8.31
- Replies: 3
- Views: 374
Re: 6th edition 8.31
Treat this problem as you would any heat capacity problem. You should be using the relations 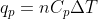 and
and 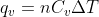 just as you would use the equation
just as you would use the equation 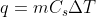 for ordinary heat capacity problems. You can find the equations for Cp and Cv on the equation sheet.
for ordinary heat capacity problems. You can find the equations for Cp and Cv on the equation sheet.
- Wed Feb 06, 2019 10:16 pm
- Forum: Phase Changes & Related Calculations
- Topic: 7th Edition 4A3
- Replies: 2
- Views: 243
Re: 7th Edition 4A3
There is a negative sign because the w, the work done by the system (the air inside the pump), is negatively proportional to its change in volume. That is, when the system expands, it does work and loses energy, and when it is compressed, work is being done on it and it gains energy.
- Wed Feb 06, 2019 10:11 pm
- Forum: Concepts & Calculations Using Second Law of Thermodynamics
- Topic: Kb and nR
- Replies: 1
- Views: 719
Re: Kb and nR
Keep in mind that in the Boltzmann equation, W (which is proportional to V) depends on the number of particles, which is in the exponent of the term inside of the ln. This term can then be moved down as a coefficient using log properties. When there is 1 mol of particles, the coefficient is N_{A} , ...
- Tue Feb 05, 2019 12:09 am
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: Finding Spontaneity of Reaction
- Replies: 1
- Views: 164
Re: Finding Spontaneity of Reaction
The state function G (Gibb's free energy) allows us to determine the spontaneity of a reaction by the relation: \Delta G^{\circ} = \Delta H^{\circ} - T\Delta S^{\circ} . When \Delta G^{\circ} is negative, the reaction is spontaneous, and when it's positive, the reaction is not spontaneous. I wouldn'...
- Tue Feb 05, 2019 12:01 am
- Forum: Third Law of Thermodynamics (For a Unique Ground State (W=1): S -> 0 as T -> 0) and Calculations Using Boltzmann Equation for Entropy
- Topic: w
- Replies: 2
- Views: 699
Re: w
Make sure you use a capital W when referring to degeneracy, since we use lowercase w to refer to work. Otherwise you will probably mix them up since they are commonly used in this unit.
- Sat Feb 02, 2019 11:22 pm
- Forum: Thermodynamic Systems (Open, Closed, Isolated)
- Topic: Biological Systems
- Replies: 3
- Views: 432
Re: Biological Systems
In biological systems, delta H and delta U are usually the same because most reactions take place in aqueous solution and not in the gas phase. Since liquids are basically incompressible/inexpandable, we can ignore the work of expansion term (PdeltaV), leaving us with deltaH=deltaU.
- Sat Feb 02, 2019 12:12 am
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: breaking double bonds?
- Replies: 6
- Views: 1310
Re: breaking double bonds?
This is because bond enthalpies are not additive; that is, the bond enthalpy of a double bond is not double that of a single bond. You can verify this by looking at the table of bond enthalpies.
- Fri Feb 01, 2019 11:55 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: 6th Edition 8.73
- Replies: 2
- Views: 344
Re: 6th Edition 8.73
You're confusing standard enthalpy of formation and bond enthalpy. The standard enthalpy of formation of Cl2(g) is 0 because that is chlorine's standard state; however, we know that bond enthalpies are never 0 because breaking bonds require energy and forming bonds release energy. Therefore, you wou...
- Fri Feb 01, 2019 11:44 pm
- Forum: Phase Changes & Related Calculations
- Topic: w = -nRTln(v2/v1)
- Replies: 6
- Views: 10854
Re: w = -nRTln(v2/v1)
You would use this equation when you have a reversible, isothermal (same temperature) expansion/compression, whereas you would use -P\Delta V for irreversible expansion at constant pressure. The derivation for the reversible process comes from the same integral: \int_{V1}^{V2}PdV , but since pressur...
- Fri Feb 01, 2019 11:25 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: 4A3
- Replies: 1
- Views: 265
Re: 4A3
When you calculate work, you typically want it to be in SI units (joules). When you just do -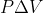 , you would get L*atm which is not SI units, so you have to convert it to joules. This conversion factor is provided on the equation sheet.
, you would get L*atm which is not SI units, so you have to convert it to joules. This conversion factor is provided on the equation sheet.
- Mon Jan 28, 2019 11:11 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: HW Week4
- Replies: 4
- Views: 492
Re: HW Week4
I would start with section 4D since for the most part covers topics such as Hess's Law and standard enthalpies of formation.
- Mon Jan 28, 2019 11:02 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Homework Problem 4A.13 7th Edition
- Replies: 2
- Views: 298
Re: Homework Problem 4A.13 7th Edition
In this problem, you would treat the calorimeter as the 'surroundings' and the reaction as the 'system.' So, if the reaction/system releases -3.50kJ of heat, then the same amount of heat is absorbed by the calorimeter/surroundings, which is a positive value. Since you are given the change in tempera...
- Mon Jan 28, 2019 10:54 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Bond Enthalpy vs Enthalpy of Formation
- Replies: 2
- Views: 384
Re: Bond Enthalpy vs Enthalpy of Formation
When you calculate the overall enthalpy of a reaction using bond enthalpies, you are not subtracting the bond enthalpies of the products from the reactants. You are summing up all the bond enthalpies. The reason why the bond enthalpies on the products side are always negative is because bonds are be...
- Mon Jan 21, 2019 6:51 pm
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Example 5J.3 Help!
- Replies: 2
- Views: 279
Re: Example 5J.3 Help!
You overlooked one very important thing, which is that H2CO3 is not a gas; it is aqueous (dissolved in water). So there is 1 mol of gas on the right and 0 on the left. Thus, compressing the system will favor the formation of H2CO3. Aqueous reactants' and products' concentrations are not affected by ...
- Mon Jan 21, 2019 6:43 pm
- Forum: Non-Equilibrium Conditions & The Reaction Quotient
- Topic: Buffer Solutions
- Replies: 2
- Views: 248
Re: Buffer Solutions
You absolutely should take the salt into consideration because it will contribute ions to solution, therefore changing the initial concentrations. The H3O+ concentration will be different than you would expect if the salt weren't there because of this! You would then solve the problem the same way u...
- Mon Jan 21, 2019 1:40 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: 12.69 (b) 6th Edition
- Replies: 1
- Views: 243
Re: 12.69 (b) 6th Edition
This reaction is given somewhere in that section in a table; since its a textbook problem you're expected to reference values and reactions that are given in the section. You know that Al3+ has to act as an acid because its a positively charged metal ion and because Cl- has no effect in water--HCl i...
- Mon Jan 21, 2019 1:34 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: 5H.2 E.g. 7th Ed
- Replies: 1
- Views: 61
Re: 5H.2 E.g. 7th Ed
They multiplied the second equation by two so that the overall reaction would be balanced. In the first equation, there is 2 PCl3 in the products, but in the second equation, there is only 1 PCl3 in the reactants. If you didn't multiply by 2, then they wouldn't cancel. Always remember to balance you...
- Mon Jan 21, 2019 1:28 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: 7th Edition Problem 6B.9 Solution
- Replies: 1
- Views: 137
Re: 7th Edition Problem 6B.9 Solution
I got the same thing. I think that it's an error in the solutions manual. A 1.0M solution of a strong acid will have a pH of 0, so we would expect a >1.0M solution of a strong acid to have a negative pH.
- Sat Jan 19, 2019 12:01 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: 6th Edition Hw #11.45 Neglible Answers
- Replies: 1
- Views: 160
Re: 6th Edition Hw #11.45 Neglible Answers
Make sure you plug in x to ALL the E parts. You will find that at least one of them will not make sense because you will have a negative concentration. For this problem, plug in x for the products; you'll see that they will have negative concentrations.
- Sat Jan 19, 2019 11:50 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Extracting the Equation from a Given question
- Replies: 1
- Views: 198
Re: Extracting the Equation from a Given question
The best way when you are given a salt solution is to break it up into its constituent ions and work from there. Typically, cations act as acids and anions act as bases. Logically, this makes sense as it would be hard for a cation to accept a positively charged proton since it itself is positively c...
- Fri Jan 18, 2019 11:32 pm
- Forum: Calculating pH or pOH for Strong & Weak Acids & Bases
- Topic: 6B.9
- Replies: 2
- Views: 282
Re: 6B.9
Actually, I think the solutions manual is wrong on this problem, and 6.667x10^-15 is correct. Since [H3O+]=1.50M which is greater than 1M (pH=0), the solution will have a negative pH (-0.176). When you were checking your work, you did 10^-0.176, but you should have done 10^0.176 because [H3O+]=10^-p...
- Fri Jan 18, 2019 11:21 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Question 6B.11 (7th Edition)
- Replies: 1
- Views: 228
Re: Question 6B.11 (7th Edition)
It'll make more sense if you write out the balanced chemical reaction: Na2O(s) + H2O(l) --> 2NaOH(aq). From this equation you can see that 1 mole of Na2O produces 2 moles of NaOH(aq), which is code for 2 moles of Na+ and OH-. Therefore the moles of Na2O is half that of OH-.
- Fri Jan 18, 2019 11:18 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: 5.61 7th Edition
- Replies: 1
- Views: 250
Re: 5.61 7th Edition
Glucose is in the aqueous state, which means it is dissolved in water. Water is incompressible, so liquid water and aqueous solutions are not affected by an increase in pressure/compression.
- Thu Jan 17, 2019 5:17 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: 6B.11
- Replies: 2
- Views: 235
Re: 6B.11
The easiest way to do this is to use the dilution formula that we used a lot in Chem 14A: since moles stay the same (n1=n2), M1V1=M2V2, where M is molarity and V is volume. Plugging in the numbers, you would get M1(5.00mL)=(0.18M)(500.0mL) --> M1=18M.
- Thu Jan 17, 2019 5:09 pm
- Forum: Non-Equilibrium Conditions & The Reaction Quotient
- Topic: 6B.11 Part B Question
- Replies: 1
- Views: 262
Re: 6B.11 Part B Question
Part B is just stoichiometry; perhaps you're confused because you haven't written out the chemical reaction: Na2O(s) + H2O(l) --> 2NaOH(aq). Using the mole ratios and the initial volume just as you would solve a stoichiometry problem, you would find that there are 1.8 moles of Na2O. If you multiply ...
- Thu Jan 17, 2019 8:25 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Why do stronger bases have conjugate acids with larger pKa values?
- Replies: 4
- Views: 2945
Re: Why do stronger bases have conjugate acids with larger pKa values?
Strong acids/bases have to have very weak conjugate bases/acids by definition because otherwise they wouldn't be strong! Strong acids/bases are not in equilibrium in solution; they dissociate completely (there is only the forward reaction). Their conjugate bases/acids are so weak that they will not ...
- Thu Jan 17, 2019 8:16 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: 7th Edition, 6B.9
- Replies: 1
- Views: 181
Re: 7th Edition, 6B.9
They give you a table with 1 of either [H30+], [OH-], pH, or pOH. You have to use the Kw or pKw formulas as well as the pH/pOH formulas to fill in the rest of the table. This should be easy as you are already given 1 value, and you are always solving for one unknown. Write down what you know in the ...
- Tue Jan 15, 2019 8:14 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: 5I.29 7th Edition
- Replies: 3
- Views: 132
Re: 5I.29 7th Edition
You most likely made an algebraic error when solving the quadratic formula. You could have avoided this altogether if you approximated that 0.22-2x ~ 0.22 since x will be really, really small (K=3.2 x 10^-34) and will basically not have a significant change on the concentration of HCl. This will mak...
- Tue Jan 15, 2019 8:10 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: 5I.23 7th Edition
- Replies: 1
- Views: 175
Re: 5I.23 7th Edition
You have to use the balanced chemical reaction in order to solve this problem, which is CO(g) + 3H2(g) <--> CH4(g) + H2O(g). I'm assuming that you remembered to divide the moles of CO and H2 by the volume of the container to get the initial concentrations of CO and H2. The initial concentrations of ...
- Tue Jan 15, 2019 8:03 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: HW problem 5I.17
- Replies: 2
- Views: 212
Re: HW problem 5I.17
The ICE box is just a great visual to see how the concentrations of the reactants and products change as the reaction reaches equilibrium. It's useful because it makes it easy to see the change in concentration based on the balanced chemical equation and gives you the expressions for the equilibrium...
- Mon Jan 14, 2019 7:44 pm
- Forum: Non-Equilibrium Conditions & The Reaction Quotient
- Topic: 7th Edition Problem 5.35
- Replies: 2
- Views: 271
Re: 7th Edition Problem 5.35
The question showed a graph of the decomposition of compound A into compounds B and C that reach equilibrium. The partial pressure is on the y-axis, in kPa. A changed from a partial pressure of 28 to 18, B from 0 to 5, and C from 0 to 10. Part (a) asked to write the balanced chemical equation for th...
- Sun Jan 13, 2019 5:01 pm
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: 6th edition, 5J11
- Replies: 2
- Views: 159
Re: 6th edition, 5J11
You could also infer that X2 is more energetically stable than X when X is a halogen because what you find in the natural world is X2, not X. If you think back to Chem 14A, halogen atoms bond to form diatomic molecules because they are more stable. The same thing goes for gases like N2 and O2 which ...
- Sun Jan 13, 2019 4:57 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Number 5I.33 7th edition
- Replies: 1
- Views: 163
Re: Number 5I.33 7th edition
I think that you forgot to convert the masses to concentrations. You have to convert to masses to moles using the molar mass and then divide by the volume to get concentration.
- Sun Jan 13, 2019 4:55 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: homework problem
- Replies: 2
- Views: 277
Re: homework problem
You have to remember to use mole ratios! Since the change in N2 is x, then the change in NO is -2x because 2 NO's react to form 1 N2 and 1H2. You get this from the balanced chemical equation.
- Sun Jan 13, 2019 4:53 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Response to Equilibria to Change
- Replies: 1
- Views: 97
Re: Response to Equilibria to Change
I think that the way that you are thinking about it is too general and therefore can be confusing. Just think about it in terms of the direction that the equilibrium shifts: if the equilibrium shifts to favor the products, then the reactants will decrease and the products will increase, and vice ver...
- Sun Jan 13, 2019 4:51 pm
- Forum: Ideal Gases
- Topic: gases
- Replies: 3
- Views: 270
Re: gases
It really depends on the information given to you in the problem. If you are given the concentrations of the gases and Kc, then use concentrations. If you are given partial pressures and K (p is usually implied in the textbook), then use partial pressures. The homework problems use both cases. This ...
- Sun Jan 13, 2019 4:48 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Molar ratios and K
- Replies: 1
- Views: 95
Re: Molar ratios and K
The power to which you raise K is equal to the coefficient by which the chemical equation is multiplied. So if you triple the equation, then the new equilibrium constant will be K^3. This general rule also explains why you square root K (raise K to the 1/2) and square K (raise K to the 2) when you h...
- Sun Jan 13, 2019 4:45 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Vid Module Part 3
- Replies: 1
- Views: 77
Re: Vid Module Part 3
Dr. Lavelle will cover this on Monday's lecture. I personally find it best to go to lecture then do the modules as review.
- Sat Jan 12, 2019 1:00 am
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: compression vs pressure
- Replies: 6
- Views: 687
Re: compression vs pressure
If the system is compressed (decreasing the volume), then the concentrations or partial pressures of the reactants and products increase, giving you a value of Q that is different from K. Comparing the value of Q to K allows us to determine which side of the reaction is favored. This is the method t...
- Sat Jan 12, 2019 12:53 am
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Adding a liquid
- Replies: 7
- Views: 562
Re: Adding a liquid
Remember that all these equilibrium reactions are all taking place in closed systems/containers. Thus, adding any solid or liquid will not change the increase the volume of the system since the volume is a fixed value. In addition, adding any solid or liquid will not decrease the volume because with...
- Sat Jan 12, 2019 12:39 am
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Endothermic
- Replies: 6
- Views: 638
Re: Endothermic
Another way to think about it is that for endothermic reactions, heat is a "reactant", and for exothermic reactions, heat is a "product." You can then apply the same concepts that we used to determine the change when reactants and products were added.
- Sat Jan 12, 2019 12:35 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Minimum and Maximum
- Replies: 2
- Views: 259
Re: Minimum and Maximum
When K approaches very large or small values, essentially what is happening is that the reaction to going to completion or isn't happening at all. Therefore the reaction is not at equilibrium and you would not have an equilibrium constant. An example that Dr. Lavelle gave is class is strong acids. S...
- Fri Jan 11, 2019 6:05 pm
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Can you cause a shift in equilibrium by increasing pressure by adding in a non-gas?
- Replies: 2
- Views: 187
Re: Can you cause a shift in equilibrium by increasing pressure by adding in a non-gas?
Most of the time, the amount of solid or liquid added is insignficant compared to the volume of the container, so adding solids or liquids would have no effect on the equilibrium. Think about it this way: 1 mole of liquid water has a volume of about 18mL at STP. If you had a 2.0L container, 18mL of ...
- Fri Jan 11, 2019 5:54 pm
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Mole Ratio with a Solid [ENDORSED]
- Replies: 3
- Views: 295
Re: Mole Ratio with a Solid [ENDORSED]
You are correct. Using the quick method, solids don't exert pressure, so decreasing the volume (and therefore increasing the pressure/concentrations of reactants) would cause the reaction to favor the formation of products by Le Chatelier's principle.
- Fri Jan 11, 2019 5:52 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Equations
- Replies: 6
- Views: 523
Re: Equations
Kc is calculated using the equilibrium molar concentrations of products and reactants while Kp is calculated using the equilibrium partial pressures of products and reactants and can only be used for gases. You can relate the two using P=(concentration)RT.
- Thu Jan 10, 2019 7:52 pm
- Forum: Non-Equilibrium Conditions & The Reaction Quotient
- Topic: Reaction quotient [ENDORSED]
- Replies: 4
- Views: 440
Re: Reaction quotient [ENDORSED]
You still have to use the reactants, otherwise you wouldn't have an expression at all! Since the products are all solid, you put a 1 for the numerator. So, your expression for the reaction quotient would be 1/[reactants in gas phase]
- Thu Jan 10, 2019 7:50 pm
- Forum: Non-Equilibrium Conditions & The Reaction Quotient
- Topic: K vs Q [ENDORSED]
- Replies: 7
- Views: 789
Re: K vs Q [ENDORSED]
The formula for K and Q is the same; the difference is that the numbers you plug in for K must be the equilibrium partial pressures or concentrations while for Q you can plug in the partial pressures or concentrations at any point in the reaction, not just at equilibrium.
- Thu Jan 10, 2019 7:46 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Help with question 11.7
- Replies: 2
- Views: 143
Re: Help with question 11.7
Pressure is directly proportional to the number of molecules/particles since all pressure really is is collisions of the particles against the container. So in this problem, there are 11 X2 molecules in the original container, so each molecule contributes a pressure of 0.10/11 bar per molecule. At e...
- Thu Jan 10, 2019 7:26 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Calculating Equilibrium Constant from Percent of Reactant
- Replies: 2
- Views: 175
Re: Calculating Equilibrium Constant from Percent of Reactant
It doesn't matter that you are only given one concentration as you can use the mole ratios in the balanced equation to find the change (and subsequently the equilibrium) concentrations of the products. This is a very similar concept to stoichiometry so think in terms of that if it makes it easier.
- Thu Jan 10, 2019 7:20 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: K vs Kp [ENDORSED]
- Replies: 4
- Views: 2933
Re: K vs Kp [ENDORSED]
I believe that since the book typically uses partial pressures for homogeneous reactions with gases, the p in Kp is implied when they give K. It would be safe to assume that if they give you a value of K and give measures of partial pressure for homogeneous reactions with gases that K=Kp.
- Thu Jan 10, 2019 7:13 pm
- Forum: Non-Equilibrium Conditions & The Reaction Quotient
- Topic: Spontaneous reverse reaction? [ENDORSED]
- Replies: 4
- Views: 400
Re: Spontaneous reverse reaction? [ENDORSED]
If a reaction is spontaneous, it just means that the reaction is thermodynamically favored. I'm sure we'll learn about this in the future when we cover thermodynamics. It would be incorrect to say that only the reverse reaction is occurring because reactions are dynamic: the forward and reverse reac...
- Tue Jan 08, 2019 8:50 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: 11.43
- Replies: 2
- Views: 243
Re: 11.43
You have to know how to use an ICE table to get the answer; however, you can still set up the expression for the equilibrium constant using the balanced equation without knowing what it is. If you don't know what an ICE table is, I would suggest waiting for Dr. Lavelle to cover it in lecture or read...
- Tue Jan 08, 2019 8:47 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: 11.41
- Replies: 1
- Views: 138
Re: 11.41
You have to use the moles of CO2 present at equilibrium to find the moles of NH3 at equilibrium and to find the moles of ammonium carbamate consumed using mole ratios. You can't use the initial moles of ammonium carbamate since the reaction does not proceed to completion (it's in equilibrium); that ...
- Tue Jan 08, 2019 8:40 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: 5G3
- Replies: 2
- Views: 167
Re: 5G3
There is a difference between the notation that you used, so yes, it does matter. Kp uses partial pressures, so you use (P C2H4CL2 )^2. Notice how when you use partial pressures, you have to use parentheses, not brackets, because brackets indicate concentration. You usually use Kp when you are given...
- Tue Jan 08, 2019 5:03 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: 7th Edition 5G.5 part c
- Replies: 2
- Views: 145
Re: 7th Edition 5G.5 part c
Using the balanced equation that Madeleine provided, you would have to find the equilibrium constant expression and then use partial pressures at equilibrium to find the value of K. I explained this in more detail in a couple other posts regarding the same question recently; you should look at those...

