Search found 66 matches
- Fri Mar 15, 2019 11:43 am
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Enthalpy At Constant Volume?
- Replies: 2
- Views: 420
Re: Enthalpy At Constant Volume?
It should, as only work is zero at constant volume. A reaction can still give off or absorb heat and the change in enthalpy will be equal to the change in internal energy.
- Fri Mar 15, 2019 10:07 am
- Forum: Ideal Gases
- Topic: How do I know what is an Ideal Gas
- Replies: 11
- Views: 974
Re: How do I know what is an Ideal Gas
The assumption is that it's an ideal gas, but questions will usually state that it's an ideal gas.
- Thu Mar 14, 2019 10:49 pm
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: Porous Wall
- Replies: 4
- Views: 670
Re: Porous Wall
I think the porous wall functions like a salt bridge in that it allows ions to move to the other electrode to even out the uneven charge distribution created by having too many electrons on one side. It's just that a porous disk tends to be used when the anode and cathode are in the same beaker and ...
- Thu Mar 14, 2019 10:30 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: ice chart
- Replies: 2
- Views: 355
Re: ice chart
The C row is for the changes in concentration. Subtract (#moles of reactants)*x from the reactants side and add (#moles of product)*x. Only do this for molecules in the aqueous or gas phase. For example, for 2NO(g) + O2(g) -> 2NO2(g), subtract 2x from the initial concentration of NO, subtract x for ...
- Thu Mar 14, 2019 10:04 pm
- Forum: Interesting Applications: Rechargeable Batteries (Cell Phones, Notebooks, Cars), Fuel Cells (Space Shuttle), Photovoltaic Cells (Solar Panels), Electrolysis, Rust
- Topic: Metal dissolution
- Replies: 10
- Views: 1030
Re: Metal dissolution
I think the metal that's oxidized and becomes an ion is the one that dissolves in solution.
- Thu Mar 07, 2019 4:54 pm
- Forum: General Rate Laws
- Topic: Reaction Order
- Replies: 1
- Views: 279
Re: Reaction Order
Order of reaction is the proportionality of concentration of reactants to rate of reaction. For example, a first order reaction's rate is proportional to the concentration of reactants raised to the power of 1. A second order reaction's rate is proportional to the concentration of reactants raised t...
- Thu Mar 07, 2019 4:38 pm
- Forum: Method of Initial Rates (To Determine n and k)
- Topic: k constant
- Replies: 2
- Views: 594
Re: k constant
Since the rate constant k is concentrations of products over reactants and you can't have a negative concentration, you can't have a negative k.
- Thu Mar 07, 2019 4:35 pm
- Forum: General Rate Laws
- Topic: Rate laws
- Replies: 2
- Views: 346
Re: Rate laws
A differential rate law always takes the form of (1/a)*(d[A]/dt)=k[A]^n while an integrated rate law takes different forms depending on the order of the reaction. For example, a first order reaction has the integrated rate law as k[A]=k[A]initial *e^-kt.
- Sat Mar 02, 2019 6:27 pm
- Forum: Work, Gibbs Free Energy, Cell (Redox) Potentials
- Topic: w vs wmax
- Replies: 5
- Views: 624
Re: w vs wmax
Maximum work (in the case of -n*F*E which is the product of total charge and cell potential) refers to the maximum amount of energy available to do work whereas work tends to be the amount of energy used (or to be used) to do work. ie Wmax is the most work that can be done with the available energy ...
- Sat Mar 02, 2019 6:18 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: H+ and OH
- Replies: 3
- Views: 434
Re: H+ and OH
I believe it's because H+ and OH- are part of the redox reaction even if they are not explicitly mentioned in the original equation (it will say whether the reaction is in a basic or acidic solution to indicate that H+ and OH- are involved).
- Sat Mar 02, 2019 6:02 pm
- Forum: Method of Initial Rates (To Determine n and k)
- Topic: Units
- Replies: 6
- Views: 728
Re: Units
Rate is typically in mol*L^-1*s*-1 or M/s where M is molarity.
- Sat Feb 23, 2019 4:46 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Cell Diagrams
- Replies: 1
- Views: 243
Re: Cell Diagrams
Ions such as K+ in KOH are used to balance charges between the cathode and anode. Because anodes are losing electrons to the cathode, the anode becomes more positively charged; we need a way to make sure the charges are equal so the reaction will keep going. This is where salt bridges and ions come ...
- Sat Feb 23, 2019 4:38 pm
- Forum: Balancing Redox Reactions
- Topic: Cell Diagram
- Replies: 2
- Views: 262
Re: Cell Diagram
An inert metal is needed because an electrode is needed for electricity/electrons to leave the anode and enter the cathode. It can be placed in either the anode or cathode, wherever a solid metal is needed (or in some cases, a liquid like mercury) as a conductor. Platinum just tends to be the main i...
- Sat Feb 23, 2019 4:28 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Driving forces
- Replies: 2
- Views: 322
Re: Driving forces
The driving force can be measured as the free energy change in a system (aka delta G). A negative delta G means there's a finite driving force for a process/reaction in the forward direction and in the reverse direction if delta G is positive. This applies to the process of dissolution as well.
- Mon Feb 18, 2019 4:40 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Gibbs Free Energy
- Replies: 5
- Views: 557
Re: Gibbs Free Energy
Since gibbs free energy is a measure of spontaneity and based on delta h and delta s, if delta h is really low (such as an exothermic reaction) it is more likely to be spontaneous and if delta s is really high (more likely to become disordered) with a high temperature it is more likely to be spontan...
- Mon Feb 18, 2019 3:48 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Meaning of subscript r
- Replies: 3
- Views: 2535
Re: Meaning of subscript r
r signals that its for a reaction, but it doesn't really change anything so don't worry about it.
- Mon Feb 18, 2019 3:43 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Compound stability with respect to decomposition
- Replies: 3
- Views: 785
Re: Compound stability with respect to decomposition
I believe because a negative delta g indicates that a reaction is spontaneous (more likely to occur or favorable), and if that compound is unstable it means that its more likely to revert back to its pure elements (hence, decomposition).
- Sun Feb 10, 2019 3:28 pm
- Forum: Entropy Changes Due to Changes in Volume and Temperature
- Topic: Delta S equations
- Replies: 3
- Views: 470
Re: Delta S equations
delta S = nCpln(T2/T1) for constant pressure and delta S = nCvln(T2/T1) for constant volume. For the values of the heat capactities, Cp is (5/2)*R and Cv is (3/2)*R for ideal gas atoms or monatomic gases. Cp=(7/2)*R and Cv=(5/2)*R for diatomic gases such as nitrogen gas (these needed to be known to ...
- Sat Feb 09, 2019 11:57 pm
- Forum: Thermodynamic Definitions (isochoric/isometric, isothermal, isobaric)
- Topic: Gibbs free energy
- Replies: 2
- Views: 313
Re: Gibbs free energy
Theoretically, gibbs free energy at a certain point is possible if given the sum of the system's enthalpy H and temperature multiplied by entropy of the system (H - T*S). This equation is very much similar to the equation for change in gibbs free energy anyways, so it is unlikely we'll have to worry...
- Sat Feb 09, 2019 11:11 pm
- Forum: Concepts & Calculations Using Second Law of Thermodynamics
- Topic: General entropy question
- Replies: 9
- Views: 848
Re: General entropy question
Entropy is higher for molecules that are in a more mobile phase (gas>liquid>solid) due to how far apart and mobile particles are relative to each other (also because q or heat is higher in that order of phases). Entropy is also higher for large, complicated molecules because there are more microstat...
- Sat Feb 02, 2019 5:38 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Heat Capactiy
- Replies: 5
- Views: 493
Re: Heat Capactiy
For example, a lake of water would require a lot more heat to raise its temperature than, say, a puddle of water. Therefore, heat capacity of the lake is greater than that of the puddle. However, specific heat capacity is the same for both because they're both bodies of water.
- Sat Feb 02, 2019 5:35 pm
- Forum: Calculating Work of Expansion
- Topic: SI unit for P
- Replies: 6
- Views: 678
Re: SI unit for P
Since atm is mostly used, the other gas constant R=.08206 L x atm/K/mol would most likely be used in questions involving PV=nRT.
- Sat Feb 02, 2019 4:24 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Current Place in Class
- Replies: 3
- Views: 384
Re: Current Place in Class
That should be the case, as the homework problems cover Thermochemistry AND the First Law of Thermodynamics.
- Sat Jan 26, 2019 3:58 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: In the last lecture
- Replies: 3
- Views: 389
Re: In the last lecture
I believe he stated that the carbon double bond breaks in the reaction and then forms a single carbon bond. Since energy is required to break a bond, the energy of a c=c bond (usually taken from a table or given) is a positive value. However, energy is released when a bond forms, so the energy of a ...
- Sat Jan 26, 2019 3:54 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Bond Enthalpies
- Replies: 3
- Views: 344
Re: Bond Enthalpies
Yes, we add up the energy of each bond that is broken (since energy is required, it's a positive number because energy is going into the system of bonds) and subtract that bonds that form (energy is released from the system). The bonds that are neither broken nor formed are not factored into the cal...
- Sat Jan 26, 2019 3:29 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: H and Q
- Replies: 4
- Views: 491
Re: H and Q
Delta H is the amount of heat absorbed/released at a constant pressure. Therefore, Qp, being quantity of heat at a constant pressure, should be essentially equal to delta H.
- Sun Jan 20, 2019 3:34 pm
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: 5% rule
- Replies: 4
- Views: 1509
Re: 5% rule
Most often, the Ka will be way less than 10^-3 (on tests and such) so as to avoid confusion on whether to approximate or not in my experience.
- Sun Jan 20, 2019 3:02 pm
- Forum: Non-Equilibrium Conditions & The Reaction Quotient
- Topic: pKa vs pH?
- Replies: 3
- Views: 427
Re: pKa vs pH?
Basically, Ka is the equilibrium constant for acid ionization (a large Ka indicates a stronger acid that has dissociated completely) and correlates with a high concentration of H+. pKa and pH are just simpler ways of expressing this by taking the -log of each.
- Thu Jan 17, 2019 10:49 pm
- Forum: Non-Equilibrium Conditions & The Reaction Quotient
- Topic: Q<K
- Replies: 4
- Views: 1299
Re: Q<K
Q and K are ratios of product to reactants. Essentially, if Q<K, then there is initially a higher ratio of reactants to products than when at equilibrium, so that means in order for Q to have the same product to reactant ratio as K, more products must be made to offset the higher reactant ratio. The...
- Mon Jan 07, 2019 8:56 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: 6th Edition 11.7 part c
- Replies: 3
- Views: 248
6th Edition 11.7 part c
Assuming that the initial pressure of X2 was 0.10 bar, calculate the value of K for the decomposition.
Can someone show me how to do this?
Can someone show me how to do this?
- Mon Jan 07, 2019 8:50 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: 6th Edition Ch11 Question 1d
- Replies: 3
- Views: 291
Re: 6th Edition Ch11 Question 1d
I believe it is due to Le Chatelier's principle (we haven't learned this yet in class, but it may be familiar from high school) where, for example, if the reactant concentration decreases, the equilibrium shifts in the direction of the reactants to restore balance, resulting in more reactants being ...
- Mon Jan 07, 2019 7:57 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Ideal Gas Law
- Replies: 1
- Views: 237
Re: Ideal Gas Law
The gas constant found on the Constants and Equations worksheet. Use R = 0.082057 atmL/molK for when pressure is in atm. Use R = 8.31446 kPaL/mol for when pressure is in kPa or kilopascals.
- Mon Jan 07, 2019 7:51 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Video Module Post Assessment Question 12
- Replies: 3
- Views: 179
Re: Video Module Post Assessment Question 12
K is the equilibrium constant and is the ratio of concentration or partial pressure of Product over Reactants (in the forward reaction) and Reactants over Products (in the reverse reaction) at equilibrium. K is associated with the reaction rate, but is different from the reaction rate because K is j...
- Sun Dec 02, 2018 3:47 pm
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: Chelates: are the rings a part of the coordination sphere?
- Replies: 2
- Views: 279
Chelates: are the rings a part of the coordination sphere?
In chelates, are the atoms that make up the rings with the ligand attached to the metal ion considered a part of the coordination sphere?
- Sun Dec 02, 2018 3:43 pm
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: How to determine coordination number
- Replies: 3
- Views: 350
Re: How to determine coordination number
Yes, it is equivalent to the number of bonds the central metal ion forms.
- Sun Dec 02, 2018 3:41 pm
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: Tetrahedral vs square planar
- Replies: 1
- Views: 311
Re: Tetrahedral vs square planar
Generally, one cannot accurately predict whether a coordination compound is square planar or tetrahedral through its coordination number alone; they would require detailed calculations or experiments. However, I do know that, almost always, metal ions in the d8 orbitals (like Ni, Pd, and Pt) will fo...
- Sun Dec 02, 2018 3:39 pm
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: structure of molecule for coordination number of 4
- Replies: 1
- Views: 168
Re: structure of molecule for coordination number of 4
Generally, one cannot accurately predict whether a coordination compound is square planar or tetrahedral through its coordination number alone; they would require detailed calculations or experiments. However, if it's worrying you a lot, almost always, metal ions in the d8 orbitals (like Ni, Pd, and...
- Sun Dec 02, 2018 3:09 pm
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: Oxidation State
- Replies: 2
- Views: 249
Re: Oxidation State
Since in coordination compounds, atoms are basically surrounding the metal ion, so to find the oxidation state of the metal ion, you'll have to look at the charges of the ligands, the coordination compound's overall charge, and atoms bonded to the compound (if any). For example, in [NiCl2(NH3)4]2H2O...
- Sun Dec 02, 2018 3:01 pm
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: Coordination Sphere
- Replies: 4
- Views: 386
Re: Coordination Sphere
If you look at coordination compounds such as [Co(NH3)6]Cl3 and [NiCl2(NH3)4]2H2O, basically anything outside of the brackets is considered outside of the coordination sphere. Structurally, the outside molecules or ions form bonds (some are more ionic in character such as the Cl- ions and some more ...
- Mon Nov 26, 2018 10:04 pm
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: NaCl vs. NiCl
- Replies: 3
- Views: 460
Re: NaCl vs. NiCl
To add on, unlike alkali metals like Na, transition metals like Ni are even smaller, highly charged cations and have many empty d-orbitals to accept electrons from groups of ions or molecules that are able to donate an electron pair(aka ligands) to form a coordinate compound.
- Mon Nov 26, 2018 9:52 pm
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: Cis and Trans
- Replies: 10
- Views: 1004
Re: Cis and Trans
If a polar bond is needed between molecules, then a cis molecule is preferred (it's polar). If the desire is to prevent polar bonds from forming, then a trans molecule is preferred (it's non-polar).
- Fri Nov 16, 2018 12:10 am
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Linear shape alternate forms
- Replies: 3
- Views: 348
Linear shape alternate forms
How is it that a linear shape can have lone pairs? Isn't it only linear when there's two areas of electron density?
- Thu Nov 15, 2018 11:27 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: bond angle
- Replies: 4
- Views: 514
Re: bond angle
90 degrees for the square shape in the middle and 180 degrees from top to bottom intersecting that square. The little video in this link may give you a better idea. https://www.sciencephoto.com/media/6014 ... lecule-sf6
- Thu Nov 15, 2018 11:20 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Repulsion Strength
- Replies: 7
- Views: 706
Re: Repulsion Strength
Qualitatively (like visually) as in a VSEPR model can predict a molecule's general shape using lone pairs and bonding pairs, but it can't predict the qualitative (numerical) aspects of a molecule that has distortions, such as bond angles. That's why, for example, a VSEPR model predicts a bent or ang...
- Sat Nov 10, 2018 4:11 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Strengths of Repulsion
- Replies: 2
- Views: 268
Re: Strengths of Repulsion
Lone pairs lie further from the central atom than bonding pairs, which are more attracted to the positive nuclei of that central atom. As such, the lone pairs are closer to each other than a lone-bonding or bonding-bonding pair and thus, experience the greatest repulsion. This in turn allows them to...
- Sat Nov 10, 2018 3:52 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Repulsion Strength
- Replies: 2
- Views: 235
Re: Repulsion Strength
The repulsion strength determines the bond angle and shape of the molecule. In molecules where there are both bond and lone pairs, lone-bonding pair's repulsion strength produces a bond angle between that atoms that is smaller than the bond angle created by an atom For example, a trigonal planar sha...
- Sat Nov 10, 2018 3:37 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Naming the shape
- Replies: 6
- Views: 649
Re: Naming the shape
Here is a chart that is quite helpful in determining the name of a shape
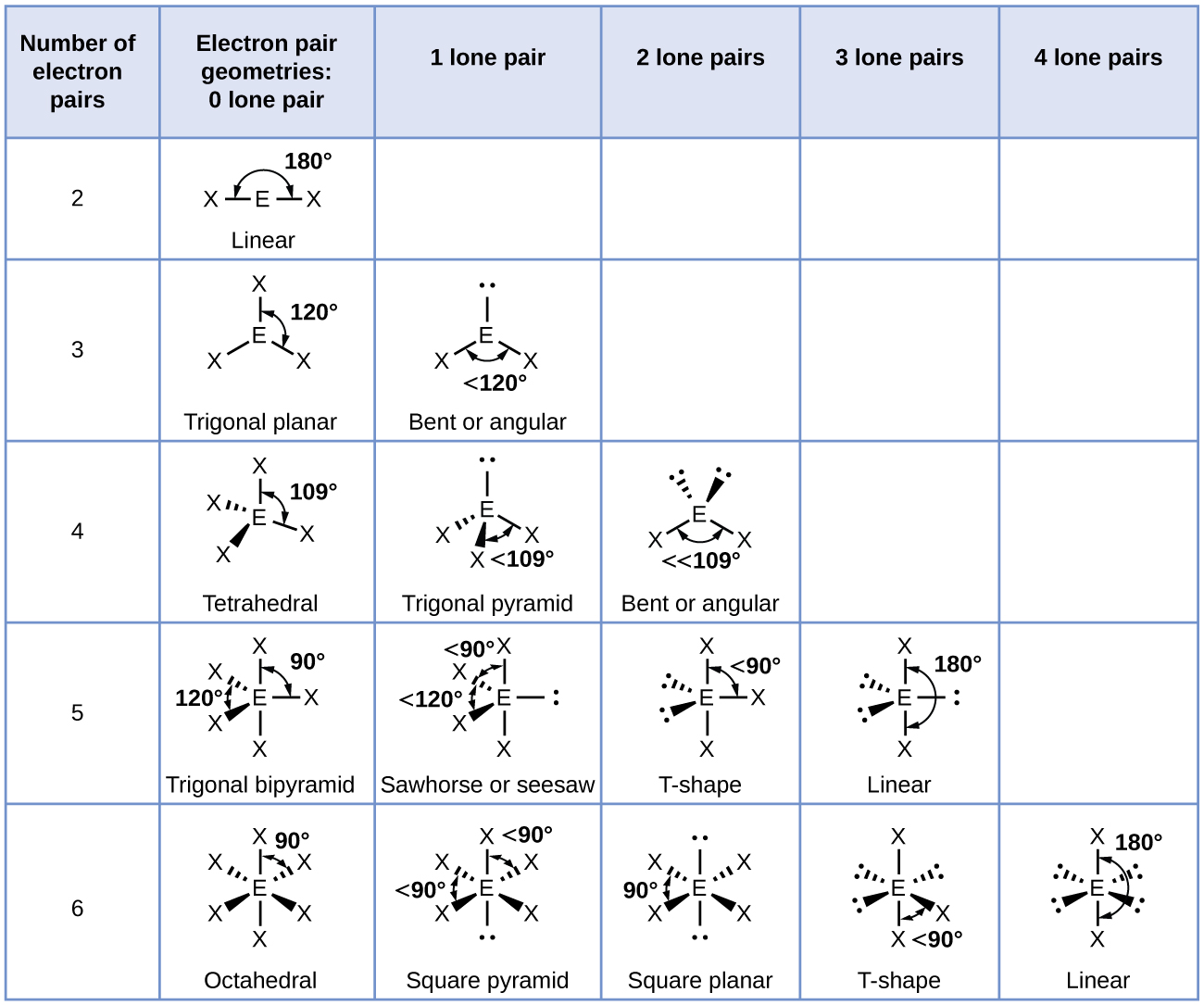

- Sat Nov 10, 2018 3:36 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Molecular Shape
- Replies: 2
- Views: 235
Re: Molecular Shape
Usually, when there are lone pairs involved instead of an atom(s), the shape will look similar, but the name and bond angles of the shape are slightly different. For example, instead of 4 atoms, a molecule may have 2 atoms and 2 lone pairs. This is called an angular or bent shape and will have bond ...
- Fri Nov 02, 2018 1:45 pm
- Forum: Sigma & Pi Bonds
- Topic: Easy way of remembering the difference
- Replies: 6
- Views: 723
Re: Easy way of remembering the difference
In special situations, a pi bond can exist between 2 atoms that don't have a net sigma-bonding effect between them, such as in certain metal complexes and some cases of atoms having multiple bonds like diiron hexacarbonyl (Fe2(CO)6). However, these are not very common and I don't believe you would h...
- Thu Nov 01, 2018 10:45 pm
- Forum: Resonance Structures
- Topic: Double Bonds and Single Bonds in Resonance Structures
- Replies: 3
- Views: 386
Re: Double Bonds and Single Bonds in Resonance Structures
Realistically, resonance structures don't actually exist; they're just a way of showing delocalized electrons where bonding cannot be expressed with just one lewis structure. Bond lengths are all experimentally the same length (the average of all the bond lengths in the atoms actually) in order to a...
- Thu Nov 01, 2018 10:25 pm
- Forum: Lewis Structures
- Topic: d orbitals in valence shell that accommodate additional e-
- Replies: 2
- Views: 174
Re: d orbitals in valence shell that accommodate additional e-
Period 3 and up elements have an empty d-orbital that may be filled so that these elements can form a more stable bond (stability seems to be the trend with bond formations) with more elements. However, elements tend to form bonds within their respective orbitals, like sulfur usually forming 2 bonds...
- Thu Nov 01, 2018 10:05 pm
- Forum: Sigma & Pi Bonds
- Topic: Easy way of remembering the difference
- Replies: 6
- Views: 723
Re: Easy way of remembering the difference
If you're asking about sigma and pi bonds, both are covalent bonds, but sigma bonds are stronger than pi bonds due to overlapping atomic orbitals (pi bonds only overlap the lobe of their atomic orbitals while sigma bonds overlap end-to-end). Generally, single bonds are always sigma bonds, but multip...
- Wed Oct 24, 2018 6:48 pm
- Forum: Trends in The Periodic Table
- Topic: Ionization Energy 2.81
- Replies: 1
- Views: 195
Re: Ionization Energy 2.81
Oxygen has 4 electrons in the 2p-orbital which is essentially an extra electron added to an already half full orbital, which results in electron electron repulsion (lowers the ionization energy). Nitrogen on the other hand, has a more stable orbital a half full 2p-orbital, so its ionization energy i...
- Mon Oct 22, 2018 5:10 pm
- Forum: Heisenberg Indeterminacy (Uncertainty) Equation
- Topic: Test 2 and Homework Problems
- Replies: 6
- Views: 665
Re: Test 2 and Homework Problems
You are correct; Test 2 covers all quantum material up to and including quantum numbers (all quantum material up to the end of Week 3. This is taken from Lavelle's website.
- Mon Oct 22, 2018 4:51 pm
- Forum: Properties of Light
- Topic: Light Intensity
- Replies: 2
- Views: 172
Re: Light Intensity
Light intensity (in this class) typically refers to the photoelectric effect in which the amount of energy need to eject electrons from a metal surface is not dependent on the intensity (number of photons) of the light, but the amount of energy per photon (E=hv), thus showing that light has properti...
- Mon Oct 15, 2018 1:29 pm
- Forum: Einstein Equation
- Topic: Kinetic energy and e = hv
- Replies: 3
- Views: 452
Re: Kinetic energy and e = hv
The concept is quite similar to escape velocity, the lowest velocity an object needs to escape gravity. The electron will barely escape the surface of the material with no leftover energy. It will continue to move; however, its speed will keep decreasing as it gets further and further, but will neve...
- Mon Oct 15, 2018 12:59 pm
- Forum: Properties of Electrons
- Topic: 7th Edition HW 1E.9
- Replies: 2
- Views: 228
Re: 7th Edition HW 1E.9
I'm assuming this is the question regarding the wavelength of a baseball of 5.15 oz moving at 92 miles/hour. The second step would be to find the the velocity in m/s by converting miles to meters (1 mile = 1609.34 m) and converting hours to seconds (1 hour = 3600 seconds). The De Broglie equations i...
- Mon Oct 15, 2018 12:49 pm
- Forum: DeBroglie Equation
- Topic: Post-Assessment Question 34
- Replies: 1
- Views: 162
Re: Post-Assessment Question 34
Using the De Broglie equation, λ=h/(mv), we should get a velocity of around 9.99x10^4 m/s. The last portion of the question is rather vague though; however, it should be noted that the speed of an electron is usually less than the speed of light (anything equal to it or above is unreasonable). A ver...
- Thu Oct 11, 2018 8:13 pm
- Forum: Properties of Electrons
- Topic: Emission vs Absorption
- Replies: 4
- Views: 406
Re: Emission vs Absorption
When an atom absorbs a specific wavelength or light, they're gaining energy so if there's enough energy, an electron will jump to a higher energy state.
- Thu Oct 11, 2018 12:08 am
- Forum: Empirical & Molecular Formulas
- Topic: Question M19: Empirical and Molecular formula of caffeine [ENDORSED]
- Replies: 4
- Views: 1590
Re: Question M19: Empirical and Molecular formula of caffeine [ENDORSED]
You want to first find mass of each element through stoichiometry using the masses of CO2, H2O, and N2 given. For example, you can find the grams of carbon (C) from grams of CO2 by finding the moles of CO2, and then finding the moles of C (it's the same moles as CO2 since both CO2 and C have only 1 ...
- Mon Oct 08, 2018 11:29 pm
- Forum: Molarity, Solutions, Dilutions
- Topic: Help with G5
- Replies: 5
- Views: 309
Re: Help with G5
Not really, it's stoichiometry, so it's cancelling out units using division and multiplication until we get the units we want. For example: [0.00251 mol Na ] [1 mol Na2CO3] [ 1 L ] -------------------------------------------------------------------------- = 1.35x10^-2 L [2 mol Na ] [0.07967 mol Na2C...
- Mon Oct 08, 2018 9:26 pm
- Forum: Empirical & Molecular Formulas
- Topic: Question L39
- Replies: 2
- Views: 387
Re: Question L39
The thing is, you want to start by finding the mass percent composition of the product to find the answer. If you convert the mass of the REACTANTS to moles, you still won't find the mole ratio of the PRODUCTS. In other words, they chose O instead of O2 because we're only focused on the empirical an...
- Sun Oct 07, 2018 4:21 pm
- Forum: Accuracy, Precision, Mole, Other Definitions
- Topic: E1
- Replies: 3
- Views: 218
Re: E1
The important thing to remember is that 1 mole is similar to 1 dozen (whereas 1 dozen is 12 of something, 1 mole is 6.022x10^23 of something). So, if it's 1 mole of atoms lined up, it's simply 6.022x10^23 atoms lined up.
- Tue Oct 02, 2018 9:13 pm
- Forum: Balancing Chemical Reactions
- Topic: Problem H. 11
- Replies: 3
- Views: 257
Re: Problem H. 11
A good tip is to write the coefficients on top of the molecules in the equation so it's easier to see how many of one element is on each side. If it doesn't balance yet, just cross off coefficients that are too small and write a larger coefficient in its place until both sides of the equation are ba...
- Tue Oct 02, 2018 8:57 pm
- Forum: Empirical & Molecular Formulas
- Topic: Fundamental L.39
- Replies: 6
- Views: 428
Re: Fundamental L.39
Okay, so you want to start by finding how much product of tin oxide you've made. We do this by subtracting the crucible's mass (26.45 g) from the total mass of the product AND the crucible (28.35 g). That should get you 1.9 g of tin oxide. And since we know we started with 1.5 g of tin, we can subtr...
- Sun Sep 30, 2018 10:38 pm
- Forum: SI Units, Unit Conversions
- Topic: Unit Conversions
- Replies: 4
- Views: 508
Re: Unit Conversions
Another helpful tip is to remember that when going from small to large units (e.g. g to kg), the numerical value should do the opposite and get smaller (e.g. 1000 g becomes 1 kg).

