The half reactions for 6N.1 (a) are
Mn2+ + 2e- = Mn (s) E = -1.18 V
Ti2+ + 2e- = Ti (s) E = -1.63 V
Why does the answer key say that Ti is the one being reduced? Shouldn't it be Mn because TI has lower reduction potential?
Search found 54 matches
- Sun Mar 17, 2019 12:33 am
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: 6N.1
- Replies: 1
- Views: 497
- Sat Mar 16, 2019 4:44 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: 7th Edition 6N.9
- Replies: 1
- Views: 284
Re: 7th Edition 6N.9
To find the pH you need to first find [H+].
To find the concentration of H+ use the equation:
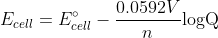
Q in the equation is equal to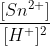
You would then solve for the concentration of H+ and then calculate pH.
To find the concentration of H+ use the equation:
Q in the equation is equal to
You would then solve for the concentration of H+ and then calculate pH.
- Sat Mar 16, 2019 4:27 pm
- Forum: Calculating pH or pOH for Strong & Weak Acids & Bases
- Topic: 6B.9
- Replies: 1
- Views: 757
Re: 6B.9
You should be able to divide Kw by the concentration of H3O+ to get the same solution for the concentration of OH-. For (i) in the table you get a negative as the answer for pH but that should also be the correct pH. pH for (i) = -0.176.
- Sat Mar 16, 2019 4:17 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: 5J.11
- Replies: 1
- Views: 189
5J.11
For question 5J.11 we are predicting which way the equilibrium shifts with a temperature increase.
(b)X2(g)⇌2X(g) where X is a halogen
The answer states that because the reaction is endothermic we know that it shifts towards products. How would we determine that this reaction is endothermic?
(b)X2(g)⇌2X(g) where X is a halogen
The answer states that because the reaction is endothermic we know that it shifts towards products. How would we determine that this reaction is endothermic?
- Sat Mar 16, 2019 1:50 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: 5I.33
- Replies: 2
- Views: 403
5I.33
The question is: A sample of ammonium carbamate, NH4(NH2CO2), of mass 25.0 g was placed in an evacuated flask of volume 0.250 L and kept at 25 °C. At equilibrium, 17.4 mg of CO2 was present. What is the value of Kc for the decomposition of ammonium carbamate into ammonia and carbon dioxide? The reac...
- Fri Mar 15, 2019 11:37 pm
- Forum: Calculating pH or pOH for Strong & Weak Acids & Bases
- Topic: Test 1 problem 5
- Replies: 6
- Views: 802
Re: Test 1 problem 5
I got pH=8.59
- Fri Mar 15, 2019 10:45 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Equillibrium Constant
- Replies: 1
- Views: 502
Re: Equillibrium Constant
If K is the equilibrium constant and every species in the reaction is multiplied by n. The new K would be K^n. K1 is the constant of chemical equation 1, K2 of equation 2, K3 of equation 3: If eq1 + eq2 = eq3, K3 = K1 x K2 If (eq1 x n) + eq2 = eq 3, K3 = (K1^n) x K2 Remember to cancel out species th...
- Fri Mar 15, 2019 10:29 pm
- Forum: Concepts & Calculations Using Second Law of Thermodynamics
- Topic: Entropy
- Replies: 3
- Views: 690
Re: Entropy
Yes.
Stotal=Ssys+Ssurr
Stotal=Ssys+Ssurr
- Fri Mar 15, 2019 10:24 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: 14.17
- Replies: 1
- Views: 249
Re: 14.17
The half-reaction for iron is actually Fe2+ -> Fe3+ + e-. Fe is being oxidized because it has a lower reduction potential.
- Fri Mar 15, 2019 10:13 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Ka Kb = Kw
- Replies: 5
- Views: 895
Re: Ka Kb = Kw
I can't be sure what will appear on the final but we used Kw on the first test. A basic reaction and the pKa was given so you had to use Kb= Kw/Ka to find the Kb to solve the problem.
- Fri Mar 15, 2019 10:10 pm
- Forum: Calculating pH or pOH for Strong & Weak Acids & Bases
- Topic: Test 1 problem 5
- Replies: 6
- Views: 802
Re: Test 1 problem 5
I have a different version of the test but I got the problem right. Could you post the full equation for the problem?
- Fri Mar 15, 2019 10:04 pm
- Forum: Reaction Mechanisms, Reaction Profiles
- Topic: Determining Rate Constant (Exercise 15.63, Sixth Edition)
- Replies: 5
- Views: 494
Re: Determining Rate Constant (Exercise 15.63, Sixth Edition)
The reason you get 0.059 is because for some reason the solutions does not convert kj to j. If you convert kj to j in the beginning, you should get 0.59.
- Fri Mar 15, 2019 9:48 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Electrolytic cell
- Replies: 2
- Views: 273
Re: Electrolytic cell
The outline doesn't really specify anything on electrolytic cells but since 6O is included in the homework problems I would mostly be concerned with the examples and homework problems.
- Fri Mar 15, 2019 8:07 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: rules of drawing the cell diagram
- Replies: 2
- Views: 316
Re: rules of drawing the cell diagram
Yes. In each half cell, reactants are first and products are last. Since the cell on the left is oxidizing, H2(g) would be before H+(aq).
- Fri Mar 15, 2019 7:58 pm
- Forum: Phase Changes & Related Calculations
- Topic: removing heat from system
- Replies: 6
- Views: 726
Re: removing heat from system
The definition of an exothermic reaction is one that releases heat.
- Mon Mar 11, 2019 4:23 am
- Forum: Balancing Redox Reactions
- Topic: Basic solution reaction
- Replies: 2
- Views: 365
Basic solution reaction
What is the reduction half-reaction for
}+ \textup{Al(s)}\rightarrow \textup{NH}_{3}\textup{(g)}+\textup{AlO}_{2}^{-}\textup{(aq)})
- Mon Mar 11, 2019 4:03 am
- Forum: Balancing Redox Reactions
- Topic: 6L.7
- Replies: 1
- Views: 233
6L.7
For 6L.7: Write the half-reactions and devise a galvanic cell (write a cell diagram) to study each of the following reactions: (a) \textup{ArBr}_{(s)}\rightleftharpoons \textup{Ag}^{+}_{aq}+\textup{Br}^{-}_{aq} How do we determine which half-reaction is oxidation and which is reduction? Why ...
- Tue Feb 12, 2019 6:16 am
- Forum: Phase Changes & Related Calculations
- Topic: Example 4B.1
- Replies: 2
- Views: 203
Example 4B.1
The example is: Engineers designing new piston engines and turbines need to understand how work and heat are involved in various compression and expansion cycles. Suppose that 1.00 mol of ideal gas molecules at an initial pressure of 3.00 atm and 292 K expands against a constant external pressure of...
- Sat Feb 02, 2019 10:35 pm
- Forum: Concepts & Calculations Using Second Law of Thermodynamics
- Topic: S and pressure
- Replies: 3
- Views: 709
Re: S and pressure
Equation for change in entropy of an ideal gas when it expands isothermally:

If initial pressure is greater than final pressure,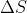 is positive.
is positive.
If initial pressure is greater than final pressure,
- Sat Feb 02, 2019 10:21 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: Delta U
- Replies: 4
- Views: 477
Re: Delta U
We are actually only measuring the changes in internal energy rather than the absolute value of the internal energy of the system.
- Sat Feb 02, 2019 10:17 pm
- Forum: Thermodynamic Definitions (isochoric/isometric, isothermal, isobaric)
- Topic: Internal Energy
- Replies: 4
- Views: 353
Re: Internal Energy
1. Start with w: w=-P_{ex}\Delta V 2. Calculate \Delta V \Delta V=1.846L-0.345L=1.501L 3. Convert the given P_{ex} from Torr to atm P_{ex}=750 \textup{ torr } \times \frac{1 \textup{ atm}}{760 \textup{ Torr}}=.99\textup{ atm} 4. Plug into equation for w w=-.99 \times 1.5 =-1.49 \textup{L}\cdot \text...
- Sun Dec 09, 2018 7:29 am
- Forum: Naming
- Topic: Churro Review- 28
- Replies: 1
- Views: 359
Churro Review- 28
Question 28 is naming the compound
Co(NH2CH2CH2NH2)2(CN)(Cl)]Cl
And the answer posted is chlorocyanobisethylenediamminecobalt (III) chloride.
Why would the Cl and CN ligands be put before the ethylenediamine ligand? Would en not be first by alphabetical order?
Co(NH2CH2CH2NH2)2(CN)(Cl)]Cl
And the answer posted is chlorocyanobisethylenediamminecobalt (III) chloride.
Why would the Cl and CN ligands be put before the ethylenediamine ligand? Would en not be first by alphabetical order?
- Sun Dec 09, 2018 6:55 am
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: Oxidation state [ENDORSED]
- Replies: 3
- Views: 755
Oxidation state [ENDORSED]
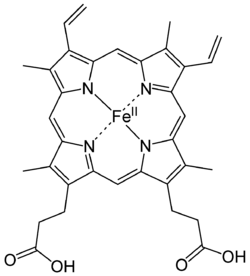
(From UA Lyndon Bui's Review)
Why is the oxidation state of the iron 2+? Shouldn't it be 4+?
- Sun Dec 09, 2018 6:48 am
- Forum: Naming
- Topic: -ate in naming
- Replies: 7
- Views: 1688
Re: -ate in naming
You don't add -ate when naming. When anions end with -ate, you change the ending from -ate to -ato to name the complex (sulfate->sulfato).
- Sun Dec 09, 2018 12:44 am
- Forum: Calculating pH or pOH for Strong & Weak Acids & Bases
- Topic: homework problem 6B.1
- Replies: 2
- Views: 326
Re: homework problem 6B.1
Assume that the pH of the initial concentration of HCl is 1.0 mol/L. Since HCl is a strong acid the concentration of H3O+ is also 1.0 mol/L pH of initial concentration = - log (1.0) = 0.00. A dilution to 12% of the initial value would make the concentration of HCl and, in turn H3O+, 0.12 mol/L. pH o...
- Sun Dec 09, 2018 12:20 am
- Forum: Hybridization
- Topic: Pi bonds
- Replies: 8
- Views: 994
Re: Pi bonds
For sp2, when there are more than 3 valence electrons in the outer shell, three will hybridize and the rest will form pi bonds. This happens because it causes no leftover elections and is optimal.
- Sun Dec 09, 2018 12:12 am
- Forum: Calculating pH or pOH for Strong & Weak Acids & Bases
- Topic: sig figs
- Replies: 4
- Views: 521
Re: sig figs
Sig figs for pH are only calculated after the decimal. For example, a ph of 4.52 would have 2 sig figs.
- Sat Dec 08, 2018 11:59 pm
- Forum: Naming
- Topic: Order of ligands
- Replies: 6
- Views: 837
Re: Order of ligands
They are put alphabetical order.
- Sat Dec 08, 2018 11:52 pm
- Forum: Conjugate Acids & Bases
- Topic: Conjugate Acids and Bases Concept
- Replies: 2
- Views: 428
Re: Conjugate Acids and Bases Concept
Yes, the acid is related to the conjugate base and the base is related to the conjugate acid.
- Sat Dec 08, 2018 11:49 pm
- Forum: Ionic & Covalent Bonds
- Topic: ionic and covalent bonds
- Replies: 5
- Views: 680
Re: ionic and covalent bonds
How are electron affinity and electronegativity the same thing? I am having a hard time understanding how the energy gained from adding an electron is related to the tendency to attract electrons.
- Sat Dec 08, 2018 11:41 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Lone pair locations
- Replies: 1
- Views: 229
Lone pair locations
In the lecture guidelines, it says "Explain why lone pairs are more likely to be found in certain locations around a central atom." Is this actually referring to their location or is it just referring to how they affect bond angles?
- Sat Dec 08, 2018 11:33 pm
- Forum: Wave Functions and s-, p-, d-, f- Orbitals
- Topic: Angular and radial nodes
- Replies: 1
- Views: 554
Angular and radial nodes
What is the difference between angular nodes and radial nodes? When a question asks for how many nodal planes exist in a certain orbital should we be answering with angular nodes?
- Sat Dec 08, 2018 11:24 pm
- Forum: Quantum Numbers and The H-Atom
- Topic: Determining electrons
- Replies: 3
- Views: 574
Re: Determining electrons
I find it easiest to think of it as shell, subshells, and orbitals.
Ex: n=2, l= 1
n = 2 means it is in the 2nd shell and l = 1 means it must be in the p orbital. There are 6 possible electrons in the 2p orbital.
Ex: n=2, l= 1
n = 2 means it is in the 2nd shell and l = 1 means it must be in the p orbital. There are 6 possible electrons in the 2p orbital.
- Sat Dec 08, 2018 11:07 pm
- Forum: Quantum Numbers and The H-Atom
- Topic: Test #2 Question #5b
- Replies: 2
- Views: 374
Re: Test #2 Question #5b
There is a nodal plane in that area and the probability of finding an election in the nodal plane is 0.
- Sat Dec 08, 2018 8:48 pm
- Forum: Calculating pH or pOH for Strong & Weak Acids & Bases
- Topic: Calculating pH or pOH with Molarity
- Replies: 3
- Views: 564
Re: Calculating pH or pOH with Molarity
Even when the molarity of a substance is given, sometimes the molarity used to calculate pH will be different because it is based on molarity of H3O+ and not the given molarity.
- Sat Dec 08, 2018 8:44 pm
- Forum: Trends in The Periodic Table
- Topic: electron affinity
- Replies: 3
- Views: 642
Re: electron affinity
Yes, but it has to be the energy released after adding an electron to a neutral atom.
- Sat Dec 08, 2018 8:39 pm
- Forum: Quantum Numbers and The H-Atom
- Topic: Representation of each Quantum Number
- Replies: 2
- Views: 552
Re: Representation of each Quantum Number
n- principal, represents the shell
l- orbital angular momentum, represents subshell in each shell
ml- magnetic, represents orbitals in subshell
ms- spin magnetic, spin state
l- orbital angular momentum, represents subshell in each shell
ml- magnetic, represents orbitals in subshell
ms- spin magnetic, spin state
- Sat Dec 08, 2018 8:24 pm
- Forum: Lewis Acids & Bases
- Topic: affects on acids
- Replies: 2
- Views: 306
Re: affects on acids
If water is used as a solvent it can act as the base. Some strong acids in water can be weak in another solvent and vice versa.
- Sat Dec 08, 2018 8:21 pm
- Forum: Lewis Acids & Bases
- Topic: acids and bases
- Replies: 2
- Views: 390
Re: acids and bases
You can't tell by the formula unless you memorize the strong acids and bases. If the Ka value is given, a larger Ka means a stronger acid.
- Sun Nov 04, 2018 8:48 pm
- Forum: Formal Charge and Oxidation Numbers
- Topic: Ideal lewis structure with Formal Charge
- Replies: 4
- Views: 2357
Ideal lewis structure with Formal Charge
How do you know what the ideal Lewis Structure is when there are many different ways of calculating the formal charge? Is it mostly trial and error?
- Sun Nov 04, 2018 8:44 pm
- Forum: Bohr Frequency Condition, H-Atom , Atomic Spectroscopy
- Topic: Fundamental and Quantum Review #6 [ENDORSED]
- Replies: 1
- Views: 427
Fundamental and Quantum Review #6 [ENDORSED]
Could someone explain the question #6 to me in UA Mr Ronald Yang's review problems and how he got to his solution again?
- Sun Nov 04, 2018 8:39 pm
- Forum: DeBroglie Equation
- Topic: Homework 1B 21
- Replies: 1
- Views: 439
Homework 1B 21
I can only get the answer to this question of 1.78E-31 m when I don't convert 92 miles to meters. Is this a mistake?
- Sun Oct 28, 2018 6:22 pm
- Forum: Electron Configurations for Multi-Electron Atoms
- Topic: Noble Gases
- Replies: 3
- Views: 352
Noble Gases
How would we write the shorthand electron configuration for noble gases?
- Sun Oct 28, 2018 6:16 pm
- Forum: Quantum Numbers and The H-Atom
- Topic: 1D.9 nodes
- Replies: 1
- Views: 314
1D.9 nodes
1D.9 asks for the number of radial nodes and angular nodal surfaces. What is the difference between these two and aren't they the same number?
- Tue Oct 23, 2018 2:17 pm
- Forum: Bohr Frequency Condition, H-Atom , Atomic Spectroscopy
- Topic: Difference in Spectroscopy [ENDORSED]
- Replies: 1
- Views: 238
Difference in Spectroscopy [ENDORSED]
What is the difference between electronic transitions in atomic orbitals and molecular orbitals?
- Sun Oct 21, 2018 8:59 pm
- Forum: Photoelectric Effect
- Topic: Conditions that allow electron to be ejected from metal surface
- Replies: 12
- Views: 2231
Re: Conditions that allow electron to be ejected from metal surface
It is also important to keep in mind the kinetic energy of the ejected electron increases linearly with the frequency of the radiation.
- Sun Oct 21, 2018 8:57 pm
- Forum: Bohr Frequency Condition, H-Atom , Atomic Spectroscopy
- Topic: Ionization
- Replies: 2
- Views: 222
Re: Ionization
The energy would be the kinetic energy of the electron (1/2mv^2).
- Tue Oct 16, 2018 9:28 pm
- Forum: Properties of Electrons
- Topic: Example 1B.3
- Replies: 2
- Views: 297
Example 1B.3
In example 1B.3 on the textbook vers 7 the step 1 to the question is
E = 1/2 * (9.109E-31 kg) * (6.68E5 ms^-1)^2
Where did they get 9.109E-31? I understand 9.109E-31 kg is the mass but I don't know where the number is from.
E = 1/2 * (9.109E-31 kg) * (6.68E5 ms^-1)^2
Where did they get 9.109E-31? I understand 9.109E-31 kg is the mass but I don't know where the number is from.
- Sun Oct 14, 2018 7:38 pm
- Forum: Properties of Light
- Topic: 1A.3 Atomic Spectra
- Replies: 2
- Views: 159
1A.3 Atomic Spectra
Can someone explain this portion of the textbook to me? "When an electric current is passed through a low-pressure sample of hydrogen gas, the gas emits light. Hydrogen gas itself does not conduct electricity, but a strong electric field strips off electrons from the H2 molecules. As a result, ...
- Sun Oct 14, 2018 7:22 pm
- Forum: Properties of Light
- Topic: Problem A9
- Replies: 5
- Views: 331
Re: Problem A9
Yes, you would first have to convert nm to m which would make it v=(3x10^8 ms^-1)/(2.5x10^-9 m).
- Sun Oct 14, 2018 7:02 pm
- Forum: Properties of Light
- Topic: Geiger and Marsden experiment
- Replies: 3
- Views: 217
Re: Geiger and Marsden experiment
Yes, some of the particles would scatter because of the nucleus making the experiments significant because it was experimental evidence that all atoms have a nucleus.
- Sun Oct 07, 2018 8:44 pm
- Forum: Limiting Reactant Calculations
- Topic: G25
- Replies: 1
- Views: 201
Re: G25
The question is basically asking whether extremely dilute solutions can affect a person. To figure this out, it asks you to double the volume 90 times and see how many molecules are left in the final solution. We start by figuring out how many molecules are in 10 ml of solution. 10ml x .10mol/1000 m...
- Sun Oct 07, 2018 8:12 pm
- Forum: Significant Figures
- Topic: Problem G5
- Replies: 2
- Views: 529
Problem G5
When I solve out G5 (b) I always end with 62.5 ml instead of 62.6 ml. Can someone explain why I can't seem to get the correct significant figures for this problem and what exactly you did to get it?
- Wed Oct 03, 2018 12:19 am
- Forum: Molarity, Solutions, Dilutions
- Topic: Question G23
- Replies: 3
- Views: 280
Re: Question G23
To expand, you would find the moles of Cl- by adding the moles of Cl from each of the compounds. To do this you would just divide the mass of each compound by the molar mass and you would not have to multiply by anything because there is only one in each.

