Search found 81 matches
- Wed Mar 13, 2019 12:20 pm
- Forum: Interesting Applications: Rechargeable Batteries (Cell Phones, Notebooks, Cars), Fuel Cells (Space Shuttle), Photovoltaic Cells (Solar Panels), Electrolysis, Rust
- Topic: Batteries
- Replies: 2
- Views: 508
Re: Batteries
Phone batteries are more complex than the examples we've been using. While a lithium-ion battery is charging, it can be considered an electrolytic cell.
- Wed Mar 13, 2019 12:17 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Gibbs free energy
- Replies: 1
- Views: 229
Re: Gibbs free energy
Writing the anode half reaction and cathode half reaction is one way to determine the number of electrons transferred. You can also just look at the charge difference between the two substances in a redox pair. In 6L.1b, a redox pair is Fe3+ and Fe2+. Since there are 6 of Fe3+ that means the charge ...
- Wed Mar 13, 2019 12:12 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: homework
- Replies: 1
- Views: 201
Re: homework
They are both hydrogen, which is always 0V because it is used set all the other values for other substances.
- Mon Mar 04, 2019 4:50 pm
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: 6N7 7th edition [ENDORSED]
- Replies: 2
- Views: 536
Re: 6N7 7th edition [ENDORSED]
I've noticed other errors concerning the value of n in the solution manual as well.
- Mon Mar 04, 2019 4:46 pm
- Forum: Zero Order Reactions
- Topic: Different Orders
- Replies: 5
- Views: 583
Re: Different Orders
Based on the assigned textbook exercises, we only need to know how to do calculations with zeroth, first, and second-order reactions.
- Mon Mar 04, 2019 4:45 pm
- Forum: General Rate Laws
- Topic: Rate Law Formula
- Replies: 2
- Views: 322
Re: Rate Law Formula
I don't think there are any exceptions for the types of reactions we will be working with in 14B.
- Tue Feb 26, 2019 9:14 am
- Forum: Work, Gibbs Free Energy, Cell (Redox) Potentials
- Topic: Problem 6N.1 Redox Reaction
- Replies: 1
- Views: 219
Re: Problem 6N.1 Redox Reaction
I assumed that was a solution manual error.
- Tue Feb 26, 2019 9:13 am
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Changes in cell potential
- Replies: 1
- Views: 220
Re: Changes in cell potential
Concentration does impact cell potential, as demonstrated by the Nernst equation.
- Mon Feb 25, 2019 10:06 am
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: When to use Platinum
- Replies: 5
- Views: 6010
Re: When to use Platinum
You add an inert electrode, such as Platinum, when the anode or cathode is lacking a metal in the solid state. For instance, when the substances in the cathode are both aqueous, Platinum could be added.
- Thu Feb 21, 2019 5:03 pm
- Forum: Balancing Redox Reactions
- Topic: Oxidation States
- Replies: 2
- Views: 258
Re: Oxidation States
The easiest way to know is by using the octet rule to figure out how many electrons the element usually gains or loses.
- Wed Feb 20, 2019 10:27 am
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Units of Gibbs Free Energy
- Replies: 5
- Views: 645
Re: Units of Gibbs Free Energy
I think Gibbs free energy is extensive because energy does depend on the amount.
- Wed Feb 20, 2019 10:25 am
- Forum: Work, Gibbs Free Energy, Cell (Redox) Potentials
- Topic: Units for Gibbs
- Replies: 8
- Views: 944
Re: Units for Gibbs
I think KJ is best, but it should not really matter.
- Wed Feb 13, 2019 1:35 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Lyndon's HOTDOG MIDTERM REVIEW SESSION!! FINALLY!
- Replies: 49
- Views: 11695
Re: Lyndon's HOTDOG MIDTERM REVIEW SESSION!! FINALLY!
For 3b: the first step is to calculate the amount of heat need to raise the temperature of all the ice cream to 0 degrees C. Then, you subtract this value from the total heat that was given. Next, you set the value for heat from the subtraction equal to the half the mass of the ice cream multiplied ...
- Wed Feb 13, 2019 9:24 am
- Forum: Phase Changes & Related Calculations
- Topic: ΔH vs ΔU?
- Replies: 8
- Views: 1397
Re: ΔH vs ΔU?
The formula for internal energy is: ΔU= q + w, so when volume is constant, w=0. Thus, when no work is done, ΔU = q. ΔH = q at constant pressure because if the pressure changed that would affect the enthalpy, but not the heat transfer.
- Tue Feb 12, 2019 10:30 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: Delta U
- Replies: 2
- Views: 344
Re: Delta U
ΔU = 0 when a process is isothermal.
- Tue Feb 12, 2019 10:21 pm
- Forum: Calculating Work of Expansion
- Topic: Uses of different formulas
- Replies: 3
- Views: 438
Re: Uses of different formulas
w= -PΔV should be used for irreversible expansions, and it is probably stated in the question if the process is irreversible. w=-nRTln(V2/V1) is for reversible expansions.
- Tue Feb 12, 2019 10:17 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Extensive v. Intensive Property of Heat Capacities
- Replies: 3
- Views: 458
Re: Extensive v. Intensive Property of Heat Capacities
Heat capacities change based on the amount of particles. Specific and molar heat capacities are for specified quantities of particles, so they are intensive.
- Tue Feb 12, 2019 10:15 pm
- Forum: Calculating the pH of Salt Solutions
- Topic: Test 1 Question 5
- Replies: 1
- Views: 748
Re: Test 1 Question 5
Since the pKa of benzoic acid is given, the first step is to find the pKb of its conjugate base (C7H5O2-). This can be found using pKa+pKb=14. Next, the Kb can be found by raising 10 to the power of -pKb. Then use an ice table, set up the Kb equation, and set the equation equal to the value found fo...
- Tue Feb 12, 2019 10:11 pm
- Forum: Third Law of Thermodynamics (For a Unique Ground State (W=1): S -> 0 as T -> 0) and Calculations Using Boltzmann Equation for Entropy
- Topic: Entropy at 0 K
- Replies: 6
- Views: 1476
Re: Entropy at 0 K
At 0K, if the structure is perfectly ordered, then entropy is equal to zero. However, if there are still possible microstates, the entropy will not be equal to zero.
- Tue Feb 12, 2019 10:08 pm
- Forum: Entropy Changes Due to Changes in Volume and Temperature
- Topic: Cv vs. Cp
- Replies: 1
- Views: 229
Re: Cv vs. Cp
Cv can be used when the entropy change is being addressed by multiple steps. For example, the entropy change can be calculated for the change in volume with ΔS=nRln(V2/V1). Then, the entropy change can be calculated for the change in temperature with ΔS=nCln(T2/T1). Since entropy is a state function...
- Tue Feb 12, 2019 10:04 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: HOTDOG #12 part B
- Replies: 6
- Views: 682
Re: HOTDOG #12 part B
When I use the equation you provided, I get 64,482.8 J, which equals the answer provided in the review session.
- Tue Feb 12, 2019 9:58 pm
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: strong and weak acids
- Replies: 5
- Views: 762
Re: strong and weak acids
Solutions of strong acids have a higher pH than solutions of weak acids of the same the molarity. For weak acids: the weaker the acid, the greater the pKa.
- Tue Feb 12, 2019 9:51 pm
- Forum: Thermodynamic Definitions (isochoric/isometric, isothermal, isobaric)
- Topic: Reversible v irreversible
- Replies: 4
- Views: 781
Re: Reversible v irreversible
If an expansion is isothermal, the change in temperature equals zero. If an expansion is reversible, w= -nRTln(V2/V1). For irreversible expansions, pressure is treated as constant. So, w= -PexΔV.
- Tue Feb 12, 2019 9:46 pm
- Forum: Phase Changes & Related Calculations
- Topic: Phase Change and Temperature
- Replies: 3
- Views: 451
Re: Phase Change and Temperature
The heat being added is energy, and that energy goes toward changing the phase, breaking bonds, not towards heating the substance.
- Tue Feb 12, 2019 9:44 pm
- Forum: Concepts & Calculations Using Second Law of Thermodynamics
- Topic: 9.13
- Replies: 2
- Views: 305
Re: 9.13
C can be substituted with Cv=(3/2)R or Cp=(5/2)R if the substance in question is an ideal gas.
- Tue Feb 12, 2019 9:26 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: HOTDOG #12 part B
- Replies: 6
- Views: 682
Re: HOTDOG #12 part B
The problem provides the change in enthalpy at 200°C, so the first step is to heat up the reactants from 37°C to 200°C. This is why the change in temperature equals 163°C. I do not see any values of 11.3 or 16.3.
- Tue Feb 12, 2019 9:19 pm
- Forum: Phase Changes & Related Calculations
- Topic: HW problem 4A. 13
- Replies: 1
- Views: 183
Re: HW problem 4A. 13
The mass of ice is in grams, specific heat capacity is in J/(°C x g), and temperature is in °C. Thus, when mCΔT is multiplied out, the units should all cancel besides Joules.
- Tue Feb 12, 2019 9:14 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: HOTDOG#7
- Replies: 2
- Views: 271
Re: HOTDOG#7
The reaction is being considered when 1.00 mol CO2 has formed. Thus, 0.5 mol PG and 0.25 mol BG reacted to form 1.00 mol CO2 and 0.25 mol H2O. The initial amount of moles was 0.75 and the final amount of moles was 1.25. Thus, delta n is 0.5 mol.
- Tue Feb 12, 2019 9:10 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Standard Reaction Enthalpy versus standard enthalpy of formation
- Replies: 1
- Views: 217
Re: Standard Reaction Enthalpy versus standard enthalpy of formation
Standard enthalpies of formation are often given so that you can solve for the standard reaction enthalpy. ΔH(rxn) =ΣΔHf(products) - ΣΔHf(reactants)
- Tue Feb 12, 2019 9:08 pm
- Forum: Calculating Work of Expansion
- Topic: when do I use 3/2R??
- Replies: 4
- Views: 2528
Re: when do I use 3/2R??
3/2R is the C value at a constant volume. It can be used in the equation ΔS=nCln(T1/T2). In this equation, C can be substituted with Cp (constant pressure) or Cv (constant volume) depending on the problem.
- Tue Feb 12, 2019 9:29 am
- Forum: Thermodynamic Definitions (isochoric/isometric, isothermal, isobaric)
- Topic: Residual energy
- Replies: 2
- Views: 464
Re: Residual energy
Do you mean residual entropy? If so, residual entropy can be best understood in comparison to residual entropy of a crystalline structure nearing 0K. The residual entropy is zero in this situation because the degeneracy (number of possible microstates^number of molecules) is equal to 1, and S=kBln(W).
- Tue Feb 12, 2019 9:21 am
- Forum: Phase Changes & Related Calculations
- Topic: Example 4B.1
- Replies: 2
- Views: 202
Re: Example 4B.1
For part b, internal energy is a state function, so although 2 different path are given, Δ U is still equal to zero. State functions are path independent.
- Tue Feb 12, 2019 9:18 am
- Forum: Phase Changes & Related Calculations
- Topic: Example 4B.1
- Replies: 2
- Views: 202
Re: Example 4B.1
Isothermal means the change in internal energy equals zero, and since Δ U = q + w, q = -w.
- Wed Feb 06, 2019 4:16 pm
- Forum: Third Law of Thermodynamics (For a Unique Ground State (W=1): S -> 0 as T -> 0) and Calculations Using Boltzmann Equation for Entropy
- Topic: S equation
- Replies: 2
- Views: 259
Re: S equation
W is degeneracy and kB is the boltzmannn constat.
- Wed Feb 06, 2019 12:24 pm
- Forum: Entropy Changes Due to Changes in Volume and Temperature
- Topic: Avogadro's Number
- Replies: 2
- Views: 301
Re: Avogadro's Number
I think the degeneracy of one mole equals 2 raised to avogadro's number because N equals the number of atoms/molecules.
- Wed Feb 06, 2019 12:17 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Ideal Gas Heat Capacity
- Replies: 1
- Views: 203
Re: Ideal Gas Heat Capacity
I think we usually are assuming ideal gas in this unit.
- Tue Jan 29, 2019 11:27 am
- Forum: Thermodynamic Systems (Open, Closed, Isolated)
- Topic: 4A.1
- Replies: 2
- Views: 224
Re: 4A.1
I just checked the solutions manual again to make sure. The mercury is a closed system, and the manual says that.
- Tue Jan 29, 2019 10:05 am
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Signs for Bond Enthalpies
- Replies: 4
- Views: 420
Re: Signs for Bond Enthalpies
It should always take energy when breaking bonds and release energy when forming bonds. However, the standard enthalpy of formation for an element is zero when it is in its standard state. For example the bond enthalpy of O2 (Oxygen) is zero.
- Tue Jan 29, 2019 10:00 am
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Enthalpy equations
- Replies: 2
- Views: 225
Re: Enthalpy equations
I do not think there are any that are excluded.
- Wed Jan 23, 2019 9:59 am
- Forum: Non-Equilibrium Conditions & The Reaction Quotient
- Topic: Acids and Bases
- Replies: 6
- Views: 562
Re: Acids and Bases
It depends on which defintion you use. A Bronsted acid donates a proton, so it must at least have a proton in the formula.
- Wed Jan 23, 2019 9:55 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: 5% Rule
- Replies: 3
- Views: 228
Re: 5% Rule
If you are given the Ka and it is very very small then sometimes it can be assumed that less than 5% of the acid was deprotonated. I think it also has to do with memorizing which acids and bases are very weak.
- Wed Jan 23, 2019 9:54 am
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Inert Gas
- Replies: 2
- Views: 185
Re: Inert Gas
I think the noble gases are inert since they have 8 valence electrons.
- Tue Jan 15, 2019 3:33 pm
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Test Next Week
- Replies: 2
- Views: 248
Re: Test Next Week
Using quizlet to remember important things is also very helpful.
- Tue Jan 15, 2019 9:21 am
- Forum: Non-Equilibrium Conditions & The Reaction Quotient
- Topic: stability
- Replies: 3
- Views: 385
Re: stability
I think it would be based on the equilibrium constant. If products are favored, they are more stable. If reactants are favored, they are more stable.
- Mon Jan 14, 2019 10:00 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Kc, Kw, and pKw
- Replies: 4
- Views: 9703
Re: Kc, Kw, and pKw
Kw = [H3O+][OH-]
- Wed Jan 09, 2019 10:24 am
- Forum: Student Social/Study Group
- Topic: New to Lavelle
- Replies: 32
- Views: 5308
Re: New to Lavelle
If you are confused about a specific topic the search tool on here is very helpful. Sometimes I'll google a question I have and the top result is this website.
- Wed Jan 09, 2019 10:21 am
- Forum: Non-Equilibrium Conditions & The Reaction Quotient
- Topic: Reaction Quotient
- Replies: 5
- Views: 432
Re: Reaction Quotient
The reaction quotient still has the same formula as the equilibirum constant, except for the equilibrium constant the values used are the ones at equilibrium.
- Wed Jan 09, 2019 10:19 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Equilibrium Constant
- Replies: 2
- Views: 132
Re: Equilibrium Constant
I think you would first convert the partial pressures to concentrations.
- Wed Dec 05, 2018 9:06 am
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: Ligands
- Replies: 4
- Views: 539
Re: Ligands
I don't think you can know without more information, such as the charge of the transition metal.
- Wed Dec 05, 2018 9:04 am
- Forum: Empirical & Molecular Formulas
- Topic: midterm question
- Replies: 3
- Views: 644
Re: midterm question
The other reactant is oxygen. So the mass of the oxygen contributes to the mass of the products.
- Wed Dec 05, 2018 9:03 am
- Forum: Amphoteric Compounds
- Topic: Amphoteric compounds
- Replies: 2
- Views: 323
Re: Amphoteric compounds
I think there are some ways to tell. For example, H2PO4-, it is apparent it can accept a proton because it has a negative charge, but it also has protons to donate.
- Sun Dec 02, 2018 8:59 pm
- Forum: Naming
- Topic: Knowing oxidation states
- Replies: 11
- Views: 954
Re: Knowing oxidation states
I think it's useful to memorize the oxidation states of the common ligands.
- Sun Dec 02, 2018 8:54 pm
- Forum: Hybridization
- Topic: P orbital
- Replies: 7
- Views: 650
Re: P orbital
what do you mean extra p orbital? sp is 2 regions of e- density, sp2 is 3 regions, sp3 is 4 regions, sp3d is 5, sp3d2 is 6.
- Sun Dec 02, 2018 8:43 pm
- Forum: Naming
- Topic: Latin prefixes
- Replies: 3
- Views: 426
Re: Latin prefixes
I think just knowing that iron is ferrate is enough.
- Mon Nov 26, 2018 4:54 pm
- Forum: Dipole Moments
- Topic: TEST 3: Polarity
- Replies: 7
- Views: 636
Re: TEST 3: Polarity
I think the arrow should always point toward the negative.
- Mon Nov 26, 2018 4:51 pm
- Forum: Hybridization
- Topic: 4.73
- Replies: 2
- Views: 356
Re: 4.73
They have to have unpaired electrons not lone pairs.
- Mon Nov 19, 2018 4:18 pm
- Forum: Hybridization
- Topic: Class 11/19/2018
- Replies: 1
- Views: 173
Re: Class 11/19/2018
Yes, it tells you how many bonds an atom can form. Which explains why atoms like carbon form hybridized orbitals.
- Mon Nov 19, 2018 3:44 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Atom Placement
- Replies: 4
- Views: 410
Re: Atom Placement
It seems that in the VSEPR model the chlorine atoms are next to each other and the oxygens are next to each other.
- Mon Nov 19, 2018 3:40 pm
- Forum: Hybridization
- Topic: Molecular Shape & Hybridization
- Replies: 4
- Views: 351
Re: Molecular Shape & Hybridization
Based on the way we took notes in class, that would make sense.
- Tue Nov 13, 2018 5:05 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Rule 2 of VSEPR Model
- Replies: 2
- Views: 260
Re: Rule 2 of VSEPR Model
Singe, double, and triple bonds have the same effect for determining the shape of a molecule. The shape is not different depending on the order of the bond.
- Tue Nov 13, 2018 4:46 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Bond Angles
- Replies: 5
- Views: 753
Re: Bond Angles
I think slightly less than has to do with lone pairs affecting what the angle normally is for a certain structure.
- Tue Nov 13, 2018 4:45 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Homework for VSEPR
- Replies: 3
- Views: 372
Re: Homework for VSEPR
Probably the questions from 2E.
- Wed Nov 07, 2018 3:28 pm
- Forum: Ionic & Covalent Bonds
- Topic: Metalloids
- Replies: 2
- Views: 291
Re: Metalloids
Covalent or Ionic bonds
- Wed Nov 07, 2018 3:27 pm
- Forum: Dipole Moments
- Topic: Dispersion strengths of larger atoms
- Replies: 5
- Views: 389
Re: Dispersion strengths of larger atoms
And more electrons mean the radius of the atom is larger.
- Wed Nov 07, 2018 3:27 pm
- Forum: Dipole Moments
- Topic: Dispersion strengths of larger atoms
- Replies: 5
- Views: 389
Re: Dispersion strengths of larger atoms
I think more electrons increases the probability of electron distortions.
- Wed Nov 07, 2018 3:25 pm
- Forum: Dipole Moments
- Topic: Intermolecular Forces
- Replies: 2
- Views: 151
Re: Intermolecular Forces
Stronger forces mean the atoms/molecules are closer together and less mobile. Thus, they are solid.
- Tue Oct 30, 2018 8:56 pm
- Forum: Lewis Structures
- Topic: determining double bonds
- Replies: 2
- Views: 271
Re: determining double bonds
I think oxygen forms two bonds typically and fluroine forms one. This is based on how many electrons they need for the octet guideline.
- Tue Oct 30, 2018 8:52 pm
- Forum: Electron Configurations for Multi-Electron Atoms
- Topic: Cu2+ configuration
- Replies: 2
- Views: 286
Re: Cu2+ configuration
It is easier to remove the outermost electrons, which are the ones in 4s.
- Tue Oct 30, 2018 8:50 pm
- Forum: Wave Functions and s-, p-, d-, f- Orbitals
- Topic: 4d^10 and 5s rule
- Replies: 2
- Views: 3356
Re: 4d^10 and 5s rule
I think it's based on the energies of those sublevels. 4d has lower energy than 5s, and the electron configurations are written in order of increasing energy.
- Tue Oct 23, 2018 2:53 pm
- Forum: Bohr Frequency Condition, H-Atom , Atomic Spectroscopy
- Topic: Rydberg Equation / Negatives on Test 2
- Replies: 1
- Views: 200
Re: Rydberg Equation / Negatives on Test 2
The equation should always be final minus initial. So E= -hR (1/nfinal2 + 1/ninitial2)
- Tue Oct 23, 2018 2:47 pm
- Forum: Einstein Equation
- Topic: λ=h/p vs λ=hc/E
- Replies: 6
- Views: 3790
Re: λ=h/p vs λ=hc/E
λ=hc/E is the same as λ=h/p except because c is used it is specific to light. λ=h/p cannot be used for light because p=mv and photons are massless.
- Tue Oct 23, 2018 2:42 pm
- Forum: Trends in The Periodic Table
- Topic: Ionization Energies
- Replies: 3
- Views: 466
Re: Ionization Energies
The first ionization energy is the energy required to remove an electron from a neutral atom (same number of protons and electrons), whereas the second ionization energy is the energy required to remove an electron from an ion with a charge of 1+. The atom is an ion because the number of electrons i...
- Tue Oct 16, 2018 5:45 pm
- Forum: *Shrodinger Equation
- Topic: Schrodinger Equation
- Replies: 3
- Views: 954
Re: Schrodinger Equation
This is Schrodinger's Equation:
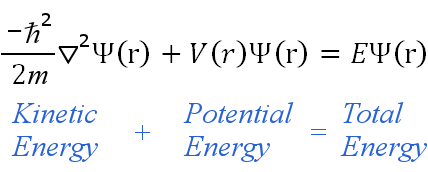
A simpler form is:
Hѱ= Eѱ
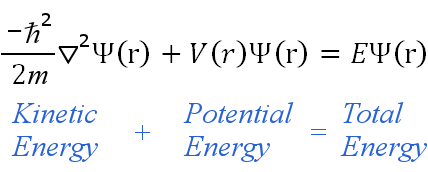
A simpler form is:
Hѱ= Eѱ
- Tue Oct 16, 2018 5:36 pm
- Forum: Student Social/Study Group
- Topic: Post All Chemistry Jokes Here
- Replies: 9651
- Views: 3591851
- Tue Oct 16, 2018 5:16 pm
- Forum: Trends in The Periodic Table
- Topic: Homework
- Replies: 10
- Views: 754
Re: Homework
I think the seven problems can come from any of the sections. For example, in my homework this week I chose to do only problems from 1D. It does not matter that we have not covered everything from this section in lecture yet.
- Sun Oct 07, 2018 6:52 pm
- Forum: Limiting Reactant Calculations
- Topic: Theoretical Yield
- Replies: 7
- Views: 949
Re: Theoretical Yield
Theoretical yield is larger than actual yield because the actual yield comes from experiments. In experiments, competing reactions may occur, the reactants may not react fully, and the reaction may not have gone to completion. Other explanations for why the maximum product was not produced are also ...
- Sun Oct 07, 2018 6:46 pm
- Forum: Significant Figures
- Topic: Sig Fig in Relation to Zero
- Replies: 3
- Views: 576
Re: Sig Fig in Relation to Zero
In 0.0490, the significant zero is only the one in the ten-thousandths place. I remember this rule because this zero shows more precision in the measuring equipment used. If the number was 0.0409, the zero between the 4 and 9 would be a significant figure.
- Sun Oct 07, 2018 6:41 pm
- Forum: Properties of Light
- Topic: Quanta & Photons
- Replies: 5
- Views: 434
Re: Quanta & Photons
I think when it says "quantized or discrete" that discrete is just serving as a definition or qualifier for the word quantized. The difference between them is not the main point. When values are quantized they become discrete.
- Thu Oct 04, 2018 8:25 pm
- Forum: Significant Figures
- Topic: Determining Sig Figs
- Replies: 6
- Views: 454
Re: Determining Sig Figs
I think it's best to use sig figs in all problems. When doing addition or subtraction, the number with the least digits after the decimal determines how many digits can be after the decimal. For Example: 1.82 + 2.1 = 3.9.
- Thu Oct 04, 2018 8:20 pm
- Forum: Molarity, Solutions, Dilutions
- Topic: Finding the Molar Mass of a metal
- Replies: 2
- Views: 145
Re: Finding the Molar Mass of a metal
I was also confused at first by this problem. The first step is to calculate the molar mass of (OH) 2 using a periodic table. Then, this value can be subtracted from the given molar mass of the metal hydroxide. This will give you the molar mass of the unknown metal. Using a periodic table you can fi...
- Thu Oct 04, 2018 8:14 pm
- Forum: SI Units, Unit Conversions
- Topic: Lecture Notes
- Replies: 2
- Views: 299
Re: Lecture Notes
Prefix : Name: Meaning: G giga 10^9 M mega 10^6 k kilo 10^3 d deci 10^(-1) c centi 10^(-2) m mili 10^(-3) μ micro 10^(-6) n nano 10^(-9) p pico 10^(-12) Volume: m^3 (extensive property: dependent on size) Density: kgm^(-3) (intensive property: independent of size) Bond lengths are 1 x 10^(-10) m, w...


