Search found 59 matches
- Sun Mar 17, 2019 10:38 pm
- Forum: Thermodynamic Definitions (isochoric/isometric, isothermal, isobaric)
- Topic: Bomb Calorimeter
- Replies: 5
- Views: 2447
Re: Bomb Calorimeter
Yes because work is equal to pressure x  V and in a bomb calorimeter, pressure and volume are constant so w=0.
V and in a bomb calorimeter, pressure and volume are constant so w=0.
- Sun Mar 17, 2019 1:03 am
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: voltaic cells
- Replies: 2
- Views: 512
Re: voltaic cells
Yes, voltaic/galvanic cells will have a salt bridge or porous disk for ion transfer to prevent charge build-up.
- Sun Mar 17, 2019 1:02 am
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: salt bridge
- Replies: 4
- Views: 727
Re: salt bridge
The salt bridge transfers ions to prevent charge build-up and allow e- transfer.
- Sat Mar 16, 2019 6:58 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Heat capacity of an object
- Replies: 1
- Views: 451
Re: Heat capacity of an object
There are different types of heat capacity.
Heat capacity is given in kJ K-1
K-1
Specific heat capacity is given in kJ g
g
 C-1, because it is referring to the amount of heat required to raise the temperature of 1 gram of the substance by 1
C-1, because it is referring to the amount of heat required to raise the temperature of 1 gram of the substance by 1 C-1.
C-1.
Heat capacity is given in kJ
Specific heat capacity is given in kJ
- Sat Mar 16, 2019 10:45 am
- Forum: General Rate Laws
- Topic: Phase of Reactants in Rate Law
- Replies: 3
- Views: 719
Re: Phase of Reactants in Rate Law
You don't include reactants that are acting as solvents. You also don't include zero order reactants but this is because they have an exponent of zero.
- Sat Mar 16, 2019 10:39 am
- Forum: Phase Changes & Related Calculations
- Topic: Bond Enthalpies
- Replies: 3
- Views: 707
Re: Bond Enthalpies
The stoichiometric coefficients correspond to the number of bonds present and when you are using bond enthalpies to calculate enthalpy you need to know how many bonds and broken or formed. So, to calculate this you need to multiply the number of bonds present in one molecule by the stoichiometric co...
- Sat Mar 16, 2019 10:31 am
- Forum: Phase Changes & Related Calculations
- Topic: moles / mass in phase change problems
- Replies: 3
- Views: 634
Re: moles / mass in phase change problems
You have to use mols in order to make sure the units cancel out with those of the gas constant.
- Wed Mar 13, 2019 10:37 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: When to put Pt(s) in a cell diagram?
- Replies: 3
- Views: 486
Re: When to put Pt(s) in a cell diagram?
You need to put a solid electrode, like Pt(s), when there are no conducting metals already present to transfer electrons.
- Wed Mar 13, 2019 10:33 pm
- Forum: Method of Initial Rates (To Determine n and k)
- Topic: Finding rate constant
- Replies: 2
- Views: 615
Finding rate constant
How do you find the rate constant for the following first-order reaction;
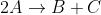 , given
, given 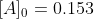 mol
mol  L-1 and that after 115s the concentration of B rises to 0.034 mol
L-1 and that after 115s the concentration of B rises to 0.034 mol  L-1.
L-1.
- Wed Mar 06, 2019 7:56 pm
- Forum: Zero Order Reactions
- Topic: Unit for rate constant for zeroth-order reaction
- Replies: 2
- Views: 479
Unit for rate constant for zeroth-order reaction
What is the unit for the rate constant for a zeroth-order reaction?
- Tue Mar 05, 2019 11:40 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Nerst Equation
- Replies: 4
- Views: 539
Re: Nerst Equation
You use the Nerst equation to find cell potential ( E_{cell} ) using the standard cell potential ( E ), gas constant ( R ), moles of electrons transferred ( n ), Faraday's constant ( F ), and the reaction quotient ( Q ). You just have to really read the question to determine what information you are...
- Tue Mar 05, 2019 11:30 pm
- Forum: General Rate Laws
- Topic: Unit for reaction rates
- Replies: 4
- Views: 483
Unit for reaction rates
When calculating the rate of a reaction, will the units include time? For example, will it be L
- Tue Mar 05, 2019 11:13 pm
- Forum: First Order Reactions
- Topic: What is the order of a reaction?
- Replies: 2
- Views: 2112
What is the order of a reaction?
Hi,
I am having trouble understanding conceptually what the order of a reaction is. What is the difference between a first and second order reaction?
I am having trouble understanding conceptually what the order of a reaction is. What is the difference between a first and second order reaction?
- Thu Feb 28, 2019 1:42 am
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Including Electrode in Cell Diagram
- Replies: 2
- Views: 292
Including Electrode in Cell Diagram
How can we tell from a chemical equation such as +I^{-}\rightarrow I_{2}(s)+Ce^{3+}(aq)) whether or not we need to put an electrode such a Pt(s) in the cell diagram?
whether or not we need to put an electrode such a Pt(s) in the cell diagram?
- Wed Feb 27, 2019 10:28 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Units
- Replies: 2
- Views: 357
Re: Units
Also remember that if you are calculating Gibbs Free Energy using a formula that has a gas constant to consider the of R, for example, R=8.314 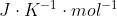
- Wed Feb 13, 2019 10:39 am
- Forum: Thermodynamic Systems (Open, Closed, Isolated)
- Topic: How Much of the Outline Do I Need to Know?
- Replies: 2
- Views: 396
Re: How Much of the Outline Do I Need to Know?
In the 7th edition, it is everything before 4J.
- Wed Feb 06, 2019 10:46 pm
- Forum: Concepts & Calculations Using Second Law of Thermodynamics
- Topic: Difference in Entropy Due to Temperature Change
- Replies: 2
- Views: 372
Difference in Entropy Due to Temperature Change
Why does change in entropy decrease when temperature increases? I know it is because in the equation 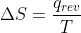
temperature is on the bottom but what is the conceptual reason behind this?
temperature is on the bottom but what is the conceptual reason behind this?
- Sun Feb 03, 2019 7:55 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: PV=nRT
- Replies: 5
- Views: 532
Re: PV=nRT
P V=
V= nRT is used when you are dealing with equations where the volume and the number of moles is changing.
nRT is used when you are dealing with equations where the volume and the number of moles is changing.
- Sun Feb 03, 2019 6:16 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: Delta U
- Replies: 7
- Views: 741
Re: Delta U
\Delta U represents the change in internal energy. A positive \Delta U tells us that the internal energy of gas has increased (usually because the temperature increased and the molecules are moving faster.) A negative \Delta U tells us that the internal energy of gas has increased (because the temp...
- Sun Feb 03, 2019 6:07 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: finding W
- Replies: 6
- Views: 636
Re: finding W
Yes -P(V2-V1 is what you get when you solve 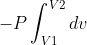
- Sun Feb 03, 2019 6:04 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: isolated vs closed system
- Replies: 7
- Views: 895
Re: isolated vs closed system
A closed system is able to exchange energy but not matter with its surroundings.
But an isolated system can exchange neither with its surroundings. For example, hot liquid in an insulated container.
But an isolated system can exchange neither with its surroundings. For example, hot liquid in an insulated container.
- Sun Feb 03, 2019 5:59 pm
- Forum: Phase Changes & Related Calculations
- Topic: Type of systems
- Replies: 12
- Views: 1339
Re: Type of systems
An isolated and a closed system are two different types of systems. A closed system can exchange energy, but not matter with its surroundings. An isolated system can't exchange matter or energy with its surroundings.
- Tue Jan 29, 2019 10:28 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: Lecture
- Replies: 5
- Views: 495
Re: Lecture
I believe you are asking about the heating curve for water that was presented in class. It illustrates that during a phase transition, there will be heat going into the reaction but there'll be no change in the temperature of the sample because all of that work is being used for the phase change.
- Sun Jan 20, 2019 7:27 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Is it an acid or a base?
- Replies: 8
- Views: 714
Re: Is it an acid or a base?
Another way you can check is by drawing a Lewis structure and checking to see if it has a lone pair to which an H+ can bond or to see if it has any hydrogen atoms that the compound can easily release.
- Sun Jan 20, 2019 7:22 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: ICE calculation sig figs
- Replies: 5
- Views: 464
Re: ICE calculation sig figs
You should not round off until the end. Only account for significant figures when you are at your final answer.
- Sun Jan 20, 2019 7:21 pm
- Forum: Non-Equilibrium Conditions & The Reaction Quotient
- Topic: Q and K
- Replies: 10
- Views: 822
Re: Q and K
Q is the reaction quotient and K is the equilibrium. Q basically tells us the ratio of the concentration or partial pressure of the products to that of the reactants when the reaction is not at equilibrium, while K tells the same thing when the reaction is at equilibrium. Comparing the two not only ...
- Sun Jan 13, 2019 8:02 pm
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Change in Pressure
- Replies: 4
- Views: 308
Re: Change in Pressure
In response to a change in pressure (due to decreased volume), a reaction will either shift to the right or the left to maintain equilibrium. A quick way to predict how a reaction will react is: if there are more moles on the right, the reaction will shift to the left and if there are more moles on ...
- Sun Jan 13, 2019 7:48 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: K vs Q
- Replies: 4
- Views: 401
Re: K vs Q
K is the equilibrium constant and is used when the reaction is in equilibrium. Q is the reaction quotient and is what we need to find when the reaction is not in equilibrium or if we are not sure if the reaction is in equilibrium.
- Sun Jan 13, 2019 7:30 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: 7th edition 5I.3
- Replies: 2
- Views: 265
Re: 7th edition 5I.3
Regarding which K value to use because this question id dealing with concentration and is asking for [H2], you will need to use Kc.
- Sat Dec 08, 2018 4:21 pm
- Forum: Dipole Moments
- Topic: Dipole Moment/Polarity
- Replies: 2
- Views: 499
Re: Dipole Moment/Polarity
A dipole moment is created when there is an unequal sharing of electrons between two atoms. You will typically see this when a difference in electronegativity between the two atoms bonded together (for example O and H). The polarity of a molecule depends on whether the dipole moments cancel out or n...
- Sat Dec 08, 2018 4:12 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: VSEPR Shape vs Molecular Geometry
- Replies: 3
- Views: 511
Re: VSEPR Shape vs Molecular Geometry
The past exams have asked for the molecular geometry. I believe if you were being asked for the VSEPR geometry it would specifically say VSEPR.
- Sat Dec 08, 2018 4:09 pm
- Forum: Naming
- Topic: adding "ion" at the end of a name
- Replies: 4
- Views: 444
Re: adding "ion" at the end of a name
You would have to add "ion" to the end of the name of a coordination complex with a positive or negative charge but not a coordination compound. The difference between these two is that a coordination complex stands alone with nothing outside of the brackets to counter the positive or nega...
- Wed Dec 05, 2018 6:15 pm
- Forum: Properties & Structures of Inorganic & Organic Acids
- Topic: Carboxyl groups and acids
- Replies: 2
- Views: 347
Re: Carboxyl groups and acids
Compounds with carboxyl group are usually acids. The H on the carboxyl group is the proton that the compound gives off in a reaction.
- Sun Dec 02, 2018 7:02 pm
- Forum: Bronsted Acids & Bases
- Topic: Bronsted vs Lewis
- Replies: 4
- Views: 482
Re: Bronsted vs Lewis
The Bronsted definitions of acids and bases relate to the protons. So a Bronsted acid is a proton donor and a Bronsted base is a proton acceptor. The Lewis definitions of acids and bases relate to the electrons. A Lewis acid is an electron acceptor and a Lewis base is an electron donor.
- Sun Dec 02, 2018 6:59 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Ozone and polarity
- Replies: 3
- Views: 11304
Re: Ozone and polarity
Ozone (O 3 ) is a polar molecule because when you draw the Lewis structure of the molecule you will see that it has a central oxygen with a lone pair of electrons, one oxygen double bonded to the central oxygen, and one oxygen single bonded to the central oxygen. This lone pair of electrons results ...
- Sun Dec 02, 2018 6:47 pm
- Forum: Interionic and Intermolecular Forces (Ion-Ion, Ion-Dipole, Dipole-Dipole, Dipole-Induced Dipole, Dispersion/Induced Dipole-Induced Dipole/London Forces, Hydrogen Bonding)
- Topic: Hydrogen Bonding
- Replies: 2
- Views: 458
Re: Hydrogen Bonding
When a molecule is able to form H-bonds with molecules around it, it typically means it is polar. The only way molecules are able to form H-bonds is when the hydrogen atoms have a slightly positive charge, which is the result of unequal sharing of electrons.
- Sun Dec 02, 2018 6:30 pm
- Forum: Ionic & Covalent Bonds
- Topic: Ionic and Covalent Character
- Replies: 3
- Views: 466
Re: Ionic and Covalent Character
Atoms that are covalently bonded usually have an electronegativity difference that is smaller and atoms that have an ionic bond have an electronegativity difference greater than 2.
- Sun Dec 02, 2018 6:08 pm
- Forum: Bronsted Acids & Bases
- Topic: Strong vs Weak Acid/Base Reactions
- Replies: 4
- Views: 431
Re: Strong vs Weak Acid/Base Reactions
I believe you simply have to memorize which acids and bases are strong and weak.
- Sun Nov 25, 2018 8:14 pm
- Forum: Hybridization
- Topic: Polar vs Non Polar
- Replies: 3
- Views: 377
Re: Polar vs Non Polar
You can determine if a molecule is polar or non-polar by looking at its symmetry. If there are dipole moments that are across from each other and cancel each other out, the molecule is non-polar. Or, if there is equal sharing of electrons between the atoms of the molecule, the molecule is polar. If,...
- Sun Nov 25, 2018 8:03 pm
- Forum: Sigma & Pi Bonds
- Topic: Why are sigma bonds stronger than pi bonds?
- Replies: 10
- Views: 4698
Re: Why are sigma bonds stronger than pi bonds?
Sigma bonds result from the head-on overlap of the orbitals of the two atoms, which has more area of overlap than pi bonds which result from the parallel overlap of orbitals. That is why sigma bonds are stronger.
- Sun Nov 25, 2018 7:55 pm
- Forum: Ionic & Covalent Bonds
- Topic: covalent bonds
- Replies: 1
- Views: 260
Re: covalent bonds
Electrons aren't typically described as distorted, bonds are described a distorted when there is a lone pair of electrons in a molecule. Because of electron repulsion, lone pairs cause all other bonds to move away from them and closer to each other, resulting in distorted bond angles that are smalle...
- Sun Nov 11, 2018 8:13 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: 7th edition 2E. 5
- Replies: 2
- Views: 254
Re: 7th edition 2E. 5
I think we can draw the structure like that with the filled in and stripped wedge. But the more important thing is naming the correct molecular shape of the molecule.
- Sun Nov 11, 2018 8:10 pm
- Forum: Ionic & Covalent Bonds
- Topic: Bond Length
- Replies: 3
- Views: 401
Re: Bond Length
I don't believe you will be expected to know specific bond lengths but you will need to know relative bond lengths. So you will need to know that single bonds are longer than double bonds and double bonds are longer than triple bonds. You will also need to know that atomic and ionic radii as well as...
- Sun Nov 11, 2018 8:03 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: tetrahedral
- Replies: 3
- Views: 389
Re: tetrahedral
I'm not sure I understand what you are asking. The VSPER shape of an electron can be tetrahedral when there is a central atom with four things bonded to it. The angles in a tetrahedral are 109.5 degrees because the four atoms bonded to the central atom want to be as far from each other due to repuls...
- Sun Nov 04, 2018 3:19 pm
- Forum: Octet Exceptions
- Topic: What are the octet exceptions?
- Replies: 9
- Views: 1128
Re: What are the octet exceptions?
H, He, Li, and Be are exceptions to the octet rule because they are smaller atoms. In additions elements in Group 13 don't necessarily need to have a full octet.
- Sun Nov 04, 2018 3:15 pm
- Forum: Formal Charge and Oxidation Numbers
- Topic: Formal Charge and Radicals
- Replies: 2
- Views: 343
Re: Formal Charge and Radicals
When we are drawing a Lewis structure for a molecule, we want the formal charges of each atom to equal 0 or to equal the overall charge of the molecule. Often times determining which atom will carry a positive or negative formal charge depends on how electronegative the element is. For example, oxyg...
- Sun Nov 04, 2018 3:04 pm
- Forum: Lewis Structures
- Topic: Octet Exceptions
- Replies: 1
- Views: 124
Re: Octet Exceptions
Group 13 elements don't need to fulfill the octet rule but they can fulfill the octet rule.
- Sun Oct 28, 2018 10:42 pm
- Forum: Electron Configurations for Multi-Electron Atoms
- Topic: Ionization of elements
- Replies: 3
- Views: 331
Re: Ionization of elements
Elements want to achieve an electron configuration that is like the closest inert gas, because this is the most stable form for the atom to be in. So, they will lose or gain just enough electrons so that they can have a full outer shell or electrons. In the case of copper, it has 2 valence electrons...
- Sun Oct 28, 2018 10:36 pm
- Forum: Ionic & Covalent Bonds
- Topic: Difference Between Ionic and Covalent Bonds
- Replies: 4
- Views: 575
Re: Difference Between Ionic and Covalent Bonds
An ionic bond usually occurs between a metal and a nonmetal, where the metal loses an electron to the nonmetal so that both atoms can be stable.
A covalent bond usually occurs between two nonmetals, where the two atoms share an electron so they can both be stable.
A covalent bond usually occurs between two nonmetals, where the two atoms share an electron so they can both be stable.
- Sun Oct 28, 2018 10:29 pm
- Forum: Trends in The Periodic Table
- Topic: s-block and p-block reactivity?
- Replies: 6
- Views: 1156
Re: s-block and p-block reactivity?
Elements in the s-block are more reactive. Yes, because they do have lower ionization energies, it is easier to remove electrons from the outer shell of these elements, making them more reactive. s-block elements usually have one or two electrons in the outer most shell, and it requires less energy ...
- Sun Oct 21, 2018 8:36 pm
- Forum: Quantum Numbers and The H-Atom
- Topic: Orbitals question
- Replies: 2
- Views: 303
Re: Orbitals question
To figure this out you want to find the first three quantum numbers, which specify the orbitals of each electron. Your principal quantum number is n =2. Using this information, you can now figure out your other quantum numbers. First you want to find l . l can be any number from 0 to ( n -1). In our...
- Sun Oct 21, 2018 8:16 pm
- Forum: Properties of Electrons
- Topic: Concept Behind Orbitals
- Replies: 6
- Views: 593
Re: Concept Behind Orbitals
Higher orbitals have more energy because the electrons have higher potential energy. If an electron is able to gain more energy it can jump to a high orbital making it easier for it to be removed from an atom. So, because the electrons in higher orbitals have higher potential energy, the orbital is ...
- Sun Oct 21, 2018 8:04 pm
- Forum: Quantum Numbers and The H-Atom
- Topic: spin part of orbitals?
- Replies: 5
- Views: 474
Re: spin part of orbitals?
The quantum number for the spin of an electron will be either +1/2 or -1/2 but I don't think we will be learning how to determine the spin of a specific electron in this class.
- Sun Oct 14, 2018 7:16 pm
- Forum: DeBroglie Equation
- Topic: De Broglie Equation Example
- Replies: 4
- Views: 462
Re: De Broglie Equation Example
Something that is very small, such as an electron, has a wavelength that is large enough to be detectable.
- Sun Oct 14, 2018 5:16 pm
- Forum: Bohr Frequency Condition, H-Atom , Atomic Spectroscopy
- Topic: E(n)=-hR/n^2
- Replies: 3
- Views: 221
Re: E(n)=-hR/n^2
The negative sign is there because a bound electron has lower energy than a free electron. So, it is there to represent the negative change in energy.
- Sun Oct 14, 2018 5:13 pm
- Forum: DeBroglie Equation
- Topic: De Broglie Equation Example
- Replies: 4
- Views: 462
Re: De Broglie Equation Example
In order for something to be considered to have wavelength properties it has to have a wavelength that is larger than n x 10^(-18) m. Anything smaller than that is not easily detectable. So, in the example you are referring to the wavelength of the car was determined to be 1.64 x 10^(-38), which is ...
- Tue Oct 02, 2018 2:00 pm
- Forum: Significant Figures
- Topic: Mass Percent Composition
- Replies: 4
- Views: 2695
Re: Mass Percent Composition
In this calculation you are typically multiplying or dividing, and the rule when it comes to significant figures in multiplication or division the rule is that your answer should have the same number of significant figures as the factor with the least number of significant figures. And also make sur...
- Tue Oct 02, 2018 1:49 pm
- Forum: Empirical & Molecular Formulas
- Topic: Determining the Number of Atoms for the Empirical Formula
- Replies: 2
- Views: 234
Determining the Number of Atoms for the Empirical Formula
Once you divide the each of the number of moles of each type of atom by the smallest value you usually get a whole number right away. But I was wondering what to do if you get a number that is slightly off, for example if you get 4.03 is it okay to use 4 for the number of atoms in the empirical form...
- Tue Oct 02, 2018 11:21 am
- Forum: Balancing Chemical Reactions
- Topic: States of Atoms
- Replies: 7
- Views: 503
Re: States of Atoms
For the time being, I would just look up the state of the atom. Professor Lavelle said the more you do these problems throughout the course of the class you will be able to remember the states of many of the molecules.

