Yes! I'm pretty sure, because it would be hard to cancel them out due to intermdiates.
for eg, take
A+catalyst-->B (fast)
B-->C + catalyst (slow)
you could cancel out for B, but then your expression would be in terms of the catalyst
Search found 66 matches
- Thu Mar 14, 2019 11:06 pm
- Forum: Reaction Mechanisms, Reaction Profiles
- Topic: Catalysts in Rate Law
- Replies: 5
- Views: 562
- Mon Mar 11, 2019 7:30 pm
- Forum: General Rate Laws
- Topic: rate law of multi step reaction
- Replies: 2
- Views: 281
Re: rate law of multi step reaction
LorenzoDuvergne3I wrote:From what I've seen, you cancel everything out that appears on both sides. Is B supposed to be the enzyme?
Yeah lol. We talked about it in a step up I went to today, but I don't think its been covered in class yet
- Mon Mar 11, 2019 7:13 pm
- Forum: General Rate Laws
- Topic: rate law of multi step reaction
- Replies: 2
- Views: 281
rate law of multi step reaction
When determining rate laws for multi step reactions, I know we have to cancel out the intermediates. However, if there is an enzyme involved
like for
A+B-->C
C-->D+B
I know we'd have to cancel for C, but would we have to for B?
Thanks!
like for
A+B-->C
C-->D+B
I know we'd have to cancel for C, but would we have to for B?
Thanks!
- Mon Mar 11, 2019 11:20 am
- Forum: General Rate Laws
- Topic: Are coefficients in rate laws?
- Replies: 2
- Views: 282
Are coefficients in rate laws?
When we have a reaction, does the stoichiometric coefficients of the reactants or products affect the rate law? How can we tell?
- Mon Mar 11, 2019 11:17 am
- Forum: Biological Examples
- Topic: Enzymes, Lecture example.
- Replies: 3
- Views: 1175
Re: Enzymes, Lecture example.
I think he was referring to an enzyme catalysed reaction, not the enzyme itself. We assume that the reactants are saturated with respect to the enzyme, so the enzymes are already working at full potential. Adding more reactant is not going to make the enzyme work faster
- Mon Mar 11, 2019 11:15 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Equilibrium Constanst
- Replies: 3
- Views: 379
Re: Equilibrium Constanst
I think for k in the rate constant you do have units, which vary depending on which order it is. But I don't think K (at equilibrium) does
- Sun Mar 03, 2019 10:50 pm
- Forum: Kinetics vs. Thermodynamics Controlling a Reaction
- Topic: how is kinetics different?
- Replies: 17
- Views: 1673
how is kinetics different?
How is kinetics different from thermodynamic? won't they both still tell which direction the reaction will proceed?
- Sun Mar 03, 2019 10:45 pm
- Forum: General Rate Laws
- Topic: Reaction Rates
- Replies: 2
- Views: 289
Re: Reaction Rates
algebraically, [NO]= 2[O2] does mean that O2 increases half as fast, because this shows that NO increases 2 times as fast as O2.
Another way you can think of it is (1/2)[NO]=[O2]
Another way you can think of it is (1/2)[NO]=[O2]
- Wed Feb 27, 2019 8:16 am
- Forum: Work, Gibbs Free Energy, Cell (Redox) Potentials
- Topic: Lyndon's Worksheet #3
- Replies: 1
- Views: 237
Re: Lyndon's Worksheet #3
So because E^o is an intensive property (does not change with amount of substance), you can't add 2 together for different reactions like you could with gibbs. This is why we have to convert to gibbs first, then add to get delta G total, then convert back to E^o total. This is separate from adding t...
- Sun Feb 24, 2019 9:43 pm
- Forum: Balancing Redox Reactions
- Topic: 2/22 in class example E(nought)
- Replies: 2
- Views: 292
2/22 in class example E(nought)
In class we had E(nought)=0.77V for Fe3+ + e- = Fe2+
When we multiplied the eqn by 2 to get 2Fe3+ + 2e- = 2Fe2+, we kept E(nought)=0.77V.
Why is this? Why didn't we multiply .77 by 2?
When we multiplied the eqn by 2 to get 2Fe3+ + 2e- = 2Fe2+, we kept E(nought)=0.77V.
Why is this? Why didn't we multiply .77 by 2?
- Sun Feb 24, 2019 9:31 pm
- Forum: Work, Gibbs Free Energy, Cell (Redox) Potentials
- Topic: What is E?
- Replies: 4
- Views: 445
What is E?
What is E (and E(nought))? In my notes I have E(nought) meaning "Standard Reaction Potential"--but I don't understand what that means, conceptually. Also, what is it used for in a battery?
- Sun Feb 24, 2019 9:24 pm
- Forum: Balancing Redox Reactions
- Topic: Salt Bridges-won't they dissolve?
- Replies: 5
- Views: 805
Salt Bridges-won't they dissolve?
I am confused. how does the salt bridge not just dissolve in the aqueous solutions on either side of the cell, making it useless?
- Sun Feb 17, 2019 10:44 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: 6th edition
- Replies: 1
- Views: 201
Re: 6th edition
I think they're from 9.51 onwards
- Tue Feb 12, 2019 5:24 pm
- Forum: Calculating Standard Reaction Entropies (e.g. , Using Standard Molar Entropies)
- Topic: 9.25 6th edition
- Replies: 4
- Views: 821
Re: 9.25 6th edition
I just worked through this one and thought to note,
because of log rules S=kbln(W^Na) is the same as S=Na*kbln(W)
It made calculating it a lot easier!
because of log rules S=kbln(W^Na) is the same as S=Na*kbln(W)
It made calculating it a lot easier!
- Tue Feb 12, 2019 9:54 am
- Forum: Calculating Standard Reaction Entropies (e.g. , Using Standard Molar Entropies)
- Topic: 9.25 6th edition
- Replies: 4
- Views: 821
9.25 6th edition
The question is:
If SO2F2 adopts a positionally disordered arrangement in its crystal form, what might its residual molar entropy be?
How would you so this? I assume use the formula S=kln(W) but I don't know how to find W in this case
If SO2F2 adopts a positionally disordered arrangement in its crystal form, what might its residual molar entropy be?
How would you so this? I assume use the formula S=kln(W) but I don't know how to find W in this case
- Sun Feb 10, 2019 8:48 pm
- Forum: Calculating Work of Expansion
- Topic: Reversible vs Irreversible
- Replies: 9
- Views: 836
Re: Reversible vs Irreversible
Can an isothermal reaction be irreversible? Or is that only for reversible reactions?
- Sun Feb 10, 2019 6:46 pm
- Forum: Calculating Work of Expansion
- Topic: Converting L*atm to J
- Replies: 2
- Views: 313
Converting L*atm to J
when using 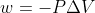 , I always get the answer in L*atm. How do you convert it to J?
, I always get the answer in L*atm. How do you convert it to J?
- Sun Feb 10, 2019 6:44 pm
- Forum: Calculating Work of Expansion
- Topic: Reversible vs Irreversible
- Replies: 9
- Views: 836
Re: Reversible vs Irreversible
So which one does more work? I think it would be reversible bc of the curve, but I'm not entirely sure why
- Wed Feb 06, 2019 1:26 pm
- Forum: Concepts & Calculations Using Second Law of Thermodynamics
- Topic: nR vs kb in entropy equations (lecture 2/6)
- Replies: 1
- Views: 232
nR vs kb in entropy equations (lecture 2/6)
Today in lecture we derived the entropy equations and related ) to
to ) .
.
What is the relationship between nR and kb?
What is the relationship between nR and kb?
- Tue Feb 05, 2019 4:50 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: 6th edition 8.51
- Replies: 2
- Views: 331
6th edition 8.51
The question gives enthalpies of formation in kJ/mol, but then asks for the enthalpy density (enthalpy released per liter). How would I find this? I am given the density of TNT in g/cm^3, but idk how i would use that
- Sun Feb 03, 2019 9:50 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: delta H vs q
- Replies: 9
- Views: 923
Re: delta H vs q
Rami_Z_AbuQubo_2K wrote:When pressure is constant, q becomes qp and this is equal to.
Why is this? How does keeping the pressure constant equal to enthalpy? Thanks!
- Sun Feb 03, 2019 9:48 pm
- Forum: Thermodynamic Systems (Open, Closed, Isolated)
- Topic: Bomb Calorimeter
- Replies: 4
- Views: 556
Re: Bomb Calorimeter
it's supposed to be an isolated system, so the chamber is insulated and the reaction happens inside, with no change of volume. Heat is measured before and after the reaction to find out how much energy was released
- Sun Feb 03, 2019 9:25 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: CV,m and CP,m
- Replies: 1
- Views: 204
Re: CV,m and CP,m
I think CP is higher because then you also have to do work to change the volume?
- Sun Jan 27, 2019 8:16 pm
- Forum: Phase Changes & Related Calculations
- Topic: 6th ed problem 8.3
- Replies: 1
- Views: 212
Re: 6th ed problem 8.3
1 cubic metre is 1000L, so I think you can just convert between the two
- Sun Jan 27, 2019 8:13 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Standard Reaction Enthalpy vs Standard Enthalpy of Formation
- Replies: 1
- Views: 197
Standard Reaction Enthalpy vs Standard Enthalpy of Formation
I am confused on the difference between Standard Reaction Enthalpy and Standard Enthalpy of Formation and how they are connected to each other. They seem to be defining the same thing which is enthalpy when all the elements are in the standard state?
- Sun Jan 27, 2019 8:06 pm
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Variables affecting pH
- Replies: 2
- Views: 252
Re: Variables affecting pH
The ones mentioned in class were all the group 1 ions (like Na+, K+), as well as Cl-. I think its because they are already pretty stable how they are, so they aren't gonna interact with the water to change the pH
- Sun Jan 20, 2019 4:21 pm
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Strong/Weak Acids and Bases
- Replies: 1
- Views: 203
Re: Strong/Weak Acids and Bases
in 14A we had to recognize strong acids/bases, but I think because we know about the Ka and Kb values this quarter we could use them instead.
- Sun Jan 20, 2019 4:13 pm
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Weak/Strong Acid and Bases
- Replies: 1
- Views: 195
Re: Weak/Strong Acid and Bases
Generally, a strong acid has Ka > 10^-3, and a weak acid has Ka < 10^-3.
a strong base has Kb > 10^-3, and a weak base has Kb < 10^-3.
Also, I think in class Lavelle mentioned that organic acids (like acetic acid) are generally weak acids.
a strong base has Kb > 10^-3, and a weak base has Kb < 10^-3.
Also, I think in class Lavelle mentioned that organic acids (like acetic acid) are generally weak acids.
- Sun Jan 20, 2019 4:10 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Calculating pH from weak base and its salt (ex. from class)
- Replies: 2
- Views: 228
Re: Calculating pH from weak base and its salt (ex. from class)
the equation is just the reaction of hno2 with water. We could use a separate one to describe the salt dissociating, but we can just infer that no2- will be there from the salt, too. Thats why for the "I" in the ICE table we put .150M NO2-, instead of 0 like we had been doing previously fo...
- Sat Jan 12, 2019 3:47 pm
- Forum: Non-Equilibrium Conditions & The Reaction Quotient
- Topic: Changing Pressure
- Replies: 6
- Views: 566
Re: Changing Pressure
Adding an inert gas wouldn't affect the equilibrium because the concentrations of the reactant and product would remain the same, meaning there would be no change in the equation. But if the volume changes, how is there not a change in the concentration if the concentration is affected by volume? T...
- Sat Jan 12, 2019 3:35 pm
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: "quick" way to solve changes in pressure
- Replies: 3
- Views: 262
"quick" way to solve changes in pressure
In class friday, Lavelle talked about a "quick" way to tell which way the reaction shifts when the volume decreases based on how many moles of gas there will be? Can someone please explain this? Does it have to do with balancing equations? In class he referred to the example N2 + 3H2 -> 2NH3
- Sat Jan 12, 2019 3:29 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Why no units for K?
- Replies: 3
- Views: 353
Why no units for K?
In class I noticed we don't have any units for K. Are there none? why?
- Sat Dec 08, 2018 8:34 pm
- Forum: Lewis Acids & Bases
- Topic: SO3 acid or base?
- Replies: 2
- Views: 4116
SO3 acid or base?
The textbook says SO3 is an acid (6th edition 12.17), and i understand that the sulfur can accept electrons, but why can't the oxygens donate their lone pairs, making it amphoteric?
- Sat Dec 08, 2018 5:27 pm
- Forum: Administrative Questions and Class Announcements
- Topic: FINAL PRACTICE - Lyndon's Churro Review Session [ENDORSED]
- Replies: 118
- Views: 21387
Re: FINAL PRACTICE - Lyndon's Churro Review Session [ENDORSED]
Can someone explain 41d - how many atoms in thymine could form a hydrogen bond. The answer is 4 but I only see 2--on either oxygen. Where are the other 2?
- Sat Dec 08, 2018 5:01 pm
- Forum: Administrative Questions and Class Announcements
- Topic: FINAL PRACTICE - Lyndon's Churro Review Session [ENDORSED]
- Replies: 118
- Views: 21387
Re: FINAL PRACTICE - Lyndon's Churro Review Session [ENDORSED]
For #31, would it also be correct to put NO2 in the complex or does it have to be ONO? I would say it has to be ONO, nitrito, because if you write NO2 that can be mistaken with nitro. Which seem similar but are not because the central atom for each differentiates. I think we should specify ONO-, bu...
- Tue Dec 04, 2018 10:30 pm
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: chelates
- Replies: 5
- Views: 537
Re: chelates
Ashita Tanwar 3H wrote:Can there be multiple rings around the same central atom?
Yes, like having 3 bidentates around one metal.
For example, you can have 3 (en) ligands around a Co, with each forming its own ring around the Co.
- Tue Dec 04, 2018 9:46 pm
- Forum: Naming
- Topic: naming differences
- Replies: 6
- Views: 634
Re: naming differences
not sure about the first one, but in one of the review sessions the TA said that chlorido and chloro meant the same thing.
- Tue Nov 27, 2018 9:43 pm
- Forum: Hybridization
- Topic: Non hybrid orbitals and pi bonds
- Replies: 1
- Views: 219
Non hybrid orbitals and pi bonds
In class, Lavelle mentioned that pi bonds form with non hybrid orbitals (the example was with C2H4).
Do pi bonds only form with non hybrid orbitals? Why?
Do pi bonds only form with non hybrid orbitals? Why?
- Tue Nov 27, 2018 9:31 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: AX3E2
- Replies: 2
- Views: 294
Re: AX3E2
So the way I think of it is, AX3E2 has 5 regions of electron density, so have the trigonal bipyramidal structure in mind (AX5). The axial bonds are 90 from each planar bond, and each planar bond is 120 from each other. To get AX3E2, we want to replace lone pairs for bonds that are furthest from the ...
- Tue Nov 27, 2018 9:11 pm
- Forum: Dipole Moments
- Topic: Dipole
- Replies: 2
- Views: 282
Re: Dipole
So a molecule has dipole moments if one atom is more electronegative than the other. we write (delta)+ next to the less electronegative atom, because the electrons are being pulled away (eg in the case of H2O, H has (delta)+) we write (delta)- next to the more electronegative atom, because the elect...
- Tue Nov 20, 2018 8:21 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Bond Angles
- Replies: 13
- Views: 2358
Re: Bond Angles
Also when can we know that bond angles are less than what they usually are? Like I remember in lecture Lavelle said a bond angle was 106.5 degrees. I think the one he was talking about was SO_3^2- (sulfite). By drawing the lewis structure, we see that the central atom, S, has 4 regions of electron ...
- Tue Nov 20, 2018 8:15 pm
- Forum: Lewis Structures
- Topic: Lewis structure for POCl3
- Replies: 7
- Views: 1369
Re: Lewis structure for POCl3
Because P has a lower ionization energy. O and Cl have roughly the same, so they all go around the outside
- Tue Nov 20, 2018 3:16 pm
- Forum: Lewis Structures
- Topic: Midterm question
- Replies: 8
- Views: 690
Re: Midterm question
I think it is sufficient to say that because of the resonance,
120pm<the N-O bond lengths<140pm.
The resonance causes the bond lengths to be longer than a double bond, but shorter than a single bond.
120pm<the N-O bond lengths<140pm.
The resonance causes the bond lengths to be longer than a double bond, but shorter than a single bond.
- Tue Nov 20, 2018 3:10 pm
- Forum: Hybridization
- Topic: Unhybridized orbitals
- Replies: 10
- Views: 1421
Re: Unhybridized orbitals
I was also confused as to whether all molecules have hybridized orbitals...I think the answer is yes, if they can. Like I assume H-H doesn't as H only has the s orbital. But from what I gather its generally necessary, at least for the central atom, so that it can bond? I think its mostly key to dete...
- Tue Nov 20, 2018 3:04 pm
- Forum: Hybridization
- Topic: hybridization and bond angles
- Replies: 2
- Views: 118
Re: hybridization and bond angles
just realized this question's already been discussed, my bad
- Tue Nov 20, 2018 3:02 pm
- Forum: Ionic & Covalent Bonds
- Topic: Silver halides & solubility
- Replies: 2
- Views: 1110
Re: Silver halides & solubility
so i'm pretty sure the silver halides are a type of salt, because they are ionic compounds. Also, I think the polarizability is how readily an anion will have a distorted electron shell (a higher polarizability means a greater covalent character). So AlF is the most soluble in water because F has th...
- Tue Nov 20, 2018 2:46 pm
- Forum: Hybridization
- Topic: hybridization and bond angles
- Replies: 2
- Views: 118
hybridization and bond angles
Does hybridization affect bond angles? I think Lavelle mentioned it in class on Monday, but I'm still confused about why hybridization relates to angles.
- Tue Nov 20, 2018 2:38 pm
- Forum: Lewis Structures
- Topic: Lewis Structure for NO3-
- Replies: 3
- Views: 3010
Lewis Structure for NO3-
How would we draw the most stable Lewis structure for NO3-?
The midterm asked why the bond lengths were the same for this structure, which suggests that they were all single or double bonds, but I can't make it work with the octets?
The midterm asked why the bond lengths were the same for this structure, which suggests that they were all single or double bonds, but I can't make it work with the octets?
- Sun Nov 11, 2018 4:53 pm
- Forum: Bond Lengths & Energies
- Topic: Negative Energy for Bonds
- Replies: 1
- Views: 148
Re: Negative Energy for Bonds
It is negative because energy is released when a bond is formed. Therefore, I think that the negative energy does imply attraction when talking about bonds because 2 atoms are forming a bond, so they're attracted to each other.
- Sun Nov 11, 2018 12:31 pm
- Forum: Dipole Moments
- Topic: Interaction potential energy, relation to distance
- Replies: 2
- Views: 288
Interaction potential energy, relation to distance
Can someone clarify the equation 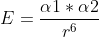
Specifically, what is meant by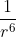 being a strong dependence?
being a strong dependence?
We talked about it during Wednesday's lecture.
Specifically, what is meant by
We talked about it during Wednesday's lecture.
- Fri Nov 09, 2018 7:40 pm
- Forum: Dipole Moments
- Topic: Hydrogen Bonds and Melting Points
- Replies: 1
- Views: 156
Re: Hydrogen Bonds and Melting Points
The reason H2O has a much higher boiling or melting point than H2S isn't down to the individual atoms, but how they interact with each other. Hydrogen bonds will generally form between molecules when the H is bonded to an N, O, or F, such as in H2O. They are weaker than covalent bonds but are still ...
- Sun Nov 04, 2018 9:21 pm
- Forum: Octet Exceptions
- Topic: Octet Exceptions
- Replies: 3
- Views: 327
Re: Octet Exceptions
H and He are exceptions because they only have the 1s orbitals, so a max of 2 electrons. Therefore, it is impossible for them to form an octet. B is an exception because it only has 3 valence electrons, so generally only forms 3 bonds (for 6 electrons), such as in BF3. However, it can form 4 bonds, ...
- Sun Nov 04, 2018 9:11 pm
- Forum: Heisenberg Indeterminacy (Uncertainty) Equation
- Topic: When to multiply by 2
- Replies: 5
- Views: 968
Re: When to multiply by 2
Do you mean like if the value is given like "the velocity is 10 +- 1 m/s"? in this case, the indeterminacy in velocity is 2, as the velocity could be between 9 and 11. So you would take the +-1 * 2.
If it says "the indeterminacy is (delta)2", then you can take that as is
If it says "the indeterminacy is (delta)2", then you can take that as is
- Sun Nov 04, 2018 5:09 pm
- Forum: Lewis Structures
- Topic: 12b from the GarBreadium worksheet
- Replies: 3
- Views: 346
Re: 12b from the GarBreadium worksheet
I think the brackets just symbolize that the molecule has a charge, like if something were []-, then the lewis structure would have an extra electron. I hope this answers your question!
- Sun Oct 28, 2018 11:00 pm
- Forum: Heisenberg Indeterminacy (Uncertainty) Equation
- Topic: Uncertainty Equation
- Replies: 2
- Views: 290
Re: Uncertainty Equation
Because uncert.p*uncert.x=h/4π, in order to maintain equilibrium, the uncertainties in p and x are inversely proportional. As in, if the uncertainty of p increases, the uncertainty of x decreases.
- Sun Oct 28, 2018 10:58 pm
- Forum: Ionic & Covalent Bonds
- Topic: Cations/Anions
- Replies: 4
- Views: 355
Re: Cations/Anions
Hi! For your first example, the whole 5p1 is removed because that represents just one electron. One electron must be removed to have a charge of +1. The opposite is true for the second example as you need 3 extra electrons
- Sun Oct 28, 2018 10:55 pm
- Forum: Lewis Structures
- Topic: Electron Configurations
- Replies: 3
- Views: 319
Re: Electron Configurations
Hi! Because to form a positive charge, 2 electronsust be removed. They are removed from the highest energy states, in this case 4s and the last 3d
- Fri Oct 19, 2018 4:44 pm
- Forum: Wave Functions and s-, p-, d-, f- Orbitals
- Topic: orbitals
- Replies: 6
- Views: 409
Re: orbitals
They represent which axis the orbital goes around, or which mathematical function is used to form it. In the case of px, the orbital goes about the x axis with the nodal plane on the zy plane.
- Fri Oct 19, 2018 4:26 pm
- Forum: Electron Configurations for Multi-Electron Atoms
- Topic: Electron Configuration Clarification
- Replies: 2
- Views: 208
Electron Configuration Clarification
In class today (Lecture 3), Lavelle said that an electron could not have the configuration (1,1,0). I am confused, why not?
- Fri Oct 19, 2018 4:13 pm
- Forum: Electron Configurations for Multi-Electron Atoms
- Topic: M quantum number
- Replies: 1
- Views: 95
Re: M quantum number
Hi!
I think that the x, y, and z is because are for p because there are 3 types of p orbitals (px, py, and pz), which we talked about on wednesday i think? So i assume for d orbitals we would call them z^2, x^2-y^2, xy, yz, and zx, which correspond to the d orbitals.
I think that the x, y, and z is because are for p because there are 3 types of p orbitals (px, py, and pz), which we talked about on wednesday i think? So i assume for d orbitals we would call them z^2, x^2-y^2, xy, yz, and zx, which correspond to the d orbitals.
- Sun Oct 14, 2018 2:21 pm
- Forum: Properties of Electrons
- Topic: Wave Length Calculations
- Replies: 5
- Views: 405
Re: Wave Length Calculations
10^-38m is not measurable simply because it is too small for us to accurately measure at this time. In class, Lavelle said that the threshold for what we could measure was around 10^-13 or 10^15m, which is still very small. For reference, the diameter of an atom is around 1 angstrom, or 10^-10m.
- Sun Oct 14, 2018 2:14 pm
- Forum: Significant Figures
- Topic: When to round the answers to significant figures
- Replies: 11
- Views: 2673
Re: When to round the answers to significant figures
It is generally more accurate to round answers only at the end, or even to manipulate/combine equations so that you only have to do one calculation to get your final answer. If you have to do multiple calculations, it is good to note extra digits in the intermediate steps so that you don't have roun...
- Sun Oct 14, 2018 2:08 pm
- Forum: Properties of Light
- Topic: De Broglie Equation
- Replies: 6
- Views: 461
Re: De Broglie Equation
Hello! In equations when you use metric units, people use kilograms instead of grams, just because kg is the standard SI unit for mass, so its what equations are based on. This is pretty conventional but its because most things we would weigh in the classical sense, like everyday objects, are better...
- Thu Oct 04, 2018 9:44 pm
- Forum: Molarity, Solutions, Dilutions
- Topic: Units on Fundamental G#19
- Replies: 1
- Views: 185
Re: Units on Fundamental G#19
Hi! The units of molarity are moles per litre, which can also be written as mol/L or mol*L^-1, because raising a number to a negative power places it on the denominator. This can be figured out using the equation M=(Moles of solute)/(Liters of solution), with the units being moles/l You can also fig...
- Thu Oct 04, 2018 9:38 pm
- Forum: Balancing Chemical Reactions
- Topic: Balancing Procedure
- Replies: 7
- Views: 779
Re: Balancing Procedure
I thought it might of been because of conservation of mass. We can't create nor destroy mass so adding it would be like creating mass? I think that's the original issue with unbalanced eqns, that there is unequal mass/numbers of elements on both sides, so by balancing them, you make it equal. in th...
- Thu Oct 04, 2018 9:29 pm
- Forum: Balancing Chemical Reactions
- Topic: Balancing Procedure
- Replies: 7
- Views: 779
Re: Balancing Procedure
Hi!
I think because singular oxygen atoms are not a proper "product" of the equation, you can't just add them on to make the equation balanced. Even though it may appear balanced, they have changed the equation when you only need to change the ratios, making the eqn incorrect.
I think because singular oxygen atoms are not a proper "product" of the equation, you can't just add them on to make the equation balanced. Even though it may appear balanced, they have changed the equation when you only need to change the ratios, making the eqn incorrect.

