Search found 29 matches
- Sat Mar 16, 2019 9:40 pm
- Forum: Reaction Mechanisms, Reaction Profiles
- Topic: Reaction rate
- Replies: 1
- Views: 490
Reaction rate
Is there an easy way to tell which reaction is fast and which is slow in a a series of reactions if we are not told in advance? Are there any tell-tale signs that give it away?
- Sat Mar 16, 2019 9:35 pm
- Forum: First Order Reactions
- Topic: Half-Lives
- Replies: 4
- Views: 839
Half-Lives
Are we suppose to ignore the half-life formulas for zero-order and second-order reactions? The textbook mentioned that we only ever really use the half-life equation of a first-order reaction, but all three formulas are on our formula sheet.
- Sat Mar 16, 2019 9:12 pm
- Forum: Reaction Mechanisms, Reaction Profiles
- Topic: net rate of reaction
- Replies: 1
- Views: 604
net rate of reaction
Is there a defined equation we use to find the net rate of reaction for a series of elementary reactions? I can't find one on our formula sheet. Or does it have to be deduced one reaction at a time? I'm just having a hard time connecting the dots between all the reactions.
- Sat Mar 16, 2019 8:32 pm
- Forum: Arrhenius Equation, Activation Energies, Catalysts
- Topic: Frequency factor
- Replies: 2
- Views: 320
Re: Frequency factor
The frequency factor is also known as the pre-exponential factor, A. It's a constant that can be derived experimentally, describing the number of times two molecules collide.
- Sat Mar 16, 2019 8:23 pm
- Forum: Arrhenius Equation, Activation Energies, Catalysts
- Topic: k(1)/k(-1)
- Replies: 4
- Views: 615
k(1)/k(-1)
What does k(1)/k(-1) mean? Why do we use it in our rate law? Mostly I just can't conceptualize what it means or how we find k(-1).
- Fri Mar 15, 2019 8:37 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Ecell
- Replies: 4
- Views: 545
Ecell
How can the Ecell of a reaction be found by both adding the Ecells of its half reactions, like Dr. Lavelle did during the review on Friday, as well as subtracting the Anode Ecell from the Cathode Ecell? Both ways seem fundamentally opposite, but yet give you the same answer. Do you change the signs ...
- Fri Mar 15, 2019 6:42 pm
- Forum: General Rate Laws
- Topic: Rate Law
- Replies: 1
- Views: 280
Rate Law
Does every reaction have a rate of formation and combustion? Or can certain rates be ignored? I feel like I've seen a problem with a reaction that didn't end up giving a rate of combustion, just formation.
- Fri Mar 15, 2019 12:32 pm
- Forum: Student Social/Study Group
- Topic: class pictures
- Replies: 15
- Views: 4049
Re: class pictures
Thanks for the help. Here you go:
- Fri Mar 15, 2019 10:50 am
- Forum: Student Social/Study Group
- Topic: class pictures
- Replies: 15
- Views: 4049
Re: class pictures
How do you post pictures? I have a decent one from up front, but I don’t know how to post it.
- Thu Mar 14, 2019 6:00 pm
- Forum: First Order Reactions
- Topic: Doubling Concentrations
- Replies: 3
- Views: 412
Doubling Concentrations
Does doubling concentrations double the order of a reaction or does it just increase the order by 1?
- Thu Mar 14, 2019 4:07 pm
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: Log vs ln
- Replies: 2
- Views: 322
Re: Log vs ln
The conversion is only done when using the second Nernst equation, however both equations mean the same thing so there is no need to convert in between them once you choose the one you are going to use.
- Thu Mar 14, 2019 4:03 pm
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: Log vs Ln
- Replies: 2
- Views: 326
Re: Log vs Ln
I'm not exactly sure how everything cancels out exactly, but I believe I can confirm from the book that there are some simplifications that can be done in order for log(Q) to be used rather than ln(Q) at the end of the equation.
- Thu Mar 14, 2019 4:00 pm
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: Nernst Equation
- Replies: 1
- Views: 309
Nernst Equation
When using the Nernst equation for concentration how does finding Q help us find the concentration we are looking for?
- Wed Mar 13, 2019 6:23 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Cell Diagram Order
- Replies: 2
- Views: 304
Cell Diagram Order
Can someone explain the order of a cell diagram better than the textbook? It's very confusing because the books states that the anode and cathode can be interchanged on the right depending on the sign if the Ecell, yet only reductions occur on the right. Earlier the text also says that the cathode i...
- Wed Mar 13, 2019 6:16 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Anode and Cathode
- Replies: 14
- Views: 1046
Anode and Cathode
How can you figure out which side of the cell diagram will be the cathode and which will be the anode? Is it only based on the whether the Ecell is negative or positive along, or are there other factors?
- Tue Mar 12, 2019 7:49 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: [tex]\Delta U = \Delta H[/tex]
- Replies: 2
- Views: 924
[tex]\Delta U = \Delta H[/tex]
I'm just a little confused when you're suppose to disregard that 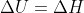 and instead use the equation
and instead use the equation 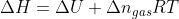 instead? What are the conditions that need to be met?
instead? What are the conditions that need to be met?
- Tue Mar 12, 2019 7:44 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Heat of combustion
- Replies: 1
- Views: 237
Re: Heat of combustion
You can solve this problem by just adding the \Delta H_{c} s correctly. In this problem all you would need to do is change -1560kJ to +1560kJ because C_{2}H_{6} is the product in this reaction. You also need to multiply -286kJ by 2 because there are two moles of H_{2} . The -1300kJ of C_{2}H_{2} doe...
- Tue Mar 12, 2019 6:19 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Heat Capacity at Constant Volume and Pressure
- Replies: 2
- Views: 523
Heat Capacity at Constant Volume and Pressure
I'm having a hard time distinguishing which constants to use in the correct situations. When do we use 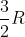 R,
R, 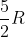 ,
, 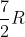 , 3R, and 4R? Are there certain ones we can ignore because they never come up, or could we be asked to use any of them on our exam?
, 3R, and 4R? Are there certain ones we can ignore because they never come up, or could we be asked to use any of them on our exam?
- Tue Mar 12, 2019 2:49 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: internal energy
- Replies: 3
- Views: 399
Re: internal energy
Shouldn't it be only isothermal, reversible reactions that have a \Delta U = 0, not irreversible? I'm just confused because example 8.5 in the book says the same about reversible, opposed to irreversible. Edit: Actually the example puts both a reversible and irreversible reactions \Delta U = 0, so I...
- Tue Mar 12, 2019 2:21 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: 4D7 temperature
- Replies: 2
- Views: 326
Re: 4D7 temperature
If I'm not mistaken the standard temperature for these reactions is  Celsius, which converts to the
Celsius, which converts to the 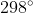 Kelvin used to solve the problem.
Kelvin used to solve the problem.
- Mon Mar 11, 2019 5:49 pm
- Forum: Ideal Gases
- Topic: Cell Diagram/Ecell
- Replies: 8
- Views: 946
Re: Cell Diagram/Ecell
Is there a difference between what is being oxidized/reduced and the oxidizing/reducing agent? I always get confused when I see one or the other in problems. In actuality do oxidizing agents get reduced and reducing agents get oxidized?
- Mon Mar 11, 2019 5:29 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Finding heat(q)
- Replies: 3
- Views: 383
Finding heat(q)
Are all the equations used to find \mathit{q} , such as \mathit{q}=C\Delta T , \mathit{q}= mC_{s}\Delta T , and \mathit{q}= nC_{s}\Delta T all just used interchangeably depending on the information provided or there certain cases where one will give you a better, or maybe more accurate answer than t...
- Mon Mar 11, 2019 5:22 pm
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: ICE
- Replies: 19
- Views: 1507
Re: ICE
E is what you are always looking to find, as it stands for Equilibrium concentration. So that line will tell you the concentrations of both your reactants and products individually as their reach their most stable state at equilibrium.
- Mon Mar 11, 2019 5:06 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Ka2
- Replies: 1
- Views: 217
Re: Ka2
I believe Ka2 is used when an acid has more than one dissociation, meaning that donates a 2nd 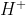
Therefore it will have more than one dissociation constant.
Therefore it will have more than one dissociation constant.
- Mon Mar 11, 2019 2:47 pm
- Forum: Ideal Gases
- Topic: 5% rule
- Replies: 12
- Views: 3470
Re: 5% rule
After reading this entire thread I've somehow only become even more confused. How can you divide the (initial condition-x)/(initial condition), if we are looking for x in the first place. There is no change in value, its still the same initial amount being divided by the initial amount, except now t...
- Sun Mar 10, 2019 4:22 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Self-Test 11.11A in textbook 6th Edition
- Replies: 1
- Views: 298
Self-Test 11.11A in textbook 6th Edition
Bromine monochloride, BrCl, decomposes into bromine and chlorine and reaches the equilibrium 2BrCl(g)\leftrightharpoons Br^{_{2}}+Cl_{2} , for which K = 32 at 500. K. If initially pure BrCl is present at a concentration of 3.30 mbar, what is its partial pressure in the mixture at equilibrium...
- Sun Mar 10, 2019 4:10 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Equation I've never seen before?
- Replies: 3
- Views: 410
Equation I've never seen before?
Are we ever going to be asked to use the equation 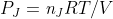 ?
?
It keeps being brought up in the examples in the book and just ends up being confusing because I don't remember if its ever been on any of our exams.
It keeps being brought up in the examples in the book and just ends up being confusing because I don't remember if its ever been on any of our exams.
- Sat Mar 09, 2019 6:56 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Negligible X
- Replies: 3
- Views: 396
Negligible X
What is the rule that can be applied when using an ice table that states you can ignore certain x values? I remember reading that it has to be less than 5% of the K value given, but always get confused when it comes to applying that rule. Something to do with 10^(-3)? Clarification would help out tr...
- Thu Feb 28, 2019 7:43 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Cell Reaction Order
- Replies: 3
- Views: 394
Cell Reaction Order
When combining half reactions you obtained from a cell diagram does it matter which side the reactants are on and which side the products are? More specifically, as long as all electrons get crossed out does the order or anything else of the final cell reaction matter? It feels like it shouldn't, bu...

