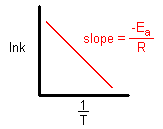
Search found 44 matches
- Sun Mar 17, 2019 4:30 pm
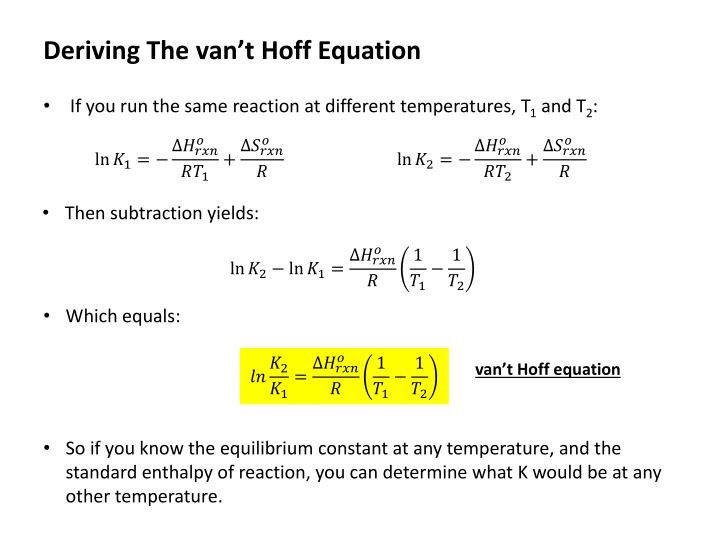
- Forum: Van't Hoff Equation
- Topic: Van't hoff
- Replies: 2
- Views: 2710
- Sun Mar 17, 2019 4:28 pm
- Forum: Arrhenius Equation, Activation Energies, Catalysts
- Topic: Catalysts
- Replies: 28
- Views: 1776
- Sun Mar 17, 2019 4:26 pm
- Forum: Arrhenius Equation, Activation Energies, Catalysts
- Topic: Plot of Arrhenius function
- Replies: 4
- Views: 799
- Sun Mar 17, 2019 4:24 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Final Grade Release Date?
- Replies: 7
- Views: 999
Final Grade Release Date?
Does anyone have an idea of when the final grades will be released?
- Sun Mar 10, 2019 9:32 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Order for Cell Diagram
- Replies: 4
- Views: 571
Re: Order for Cell Diagram
In addition, my ta told me that Lavelle prefers you put the aqueous elements closer to the double line (salt bridge) in the middle, and platinum (if included) on the outside.
- Sun Mar 10, 2019 9:29 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Final Questions
- Replies: 10
- Views: 1212
Re: Final Questions
Considering the midterm and past finals, it will probably be somewhere between 8 to 10 questions.
- Sun Mar 10, 2019 9:25 pm
- Forum: Zero Order Reactions
- Topic: Graphs
- Replies: 8
- Views: 2213
Re: Graphs
Here is a diagram that you might find helpful:
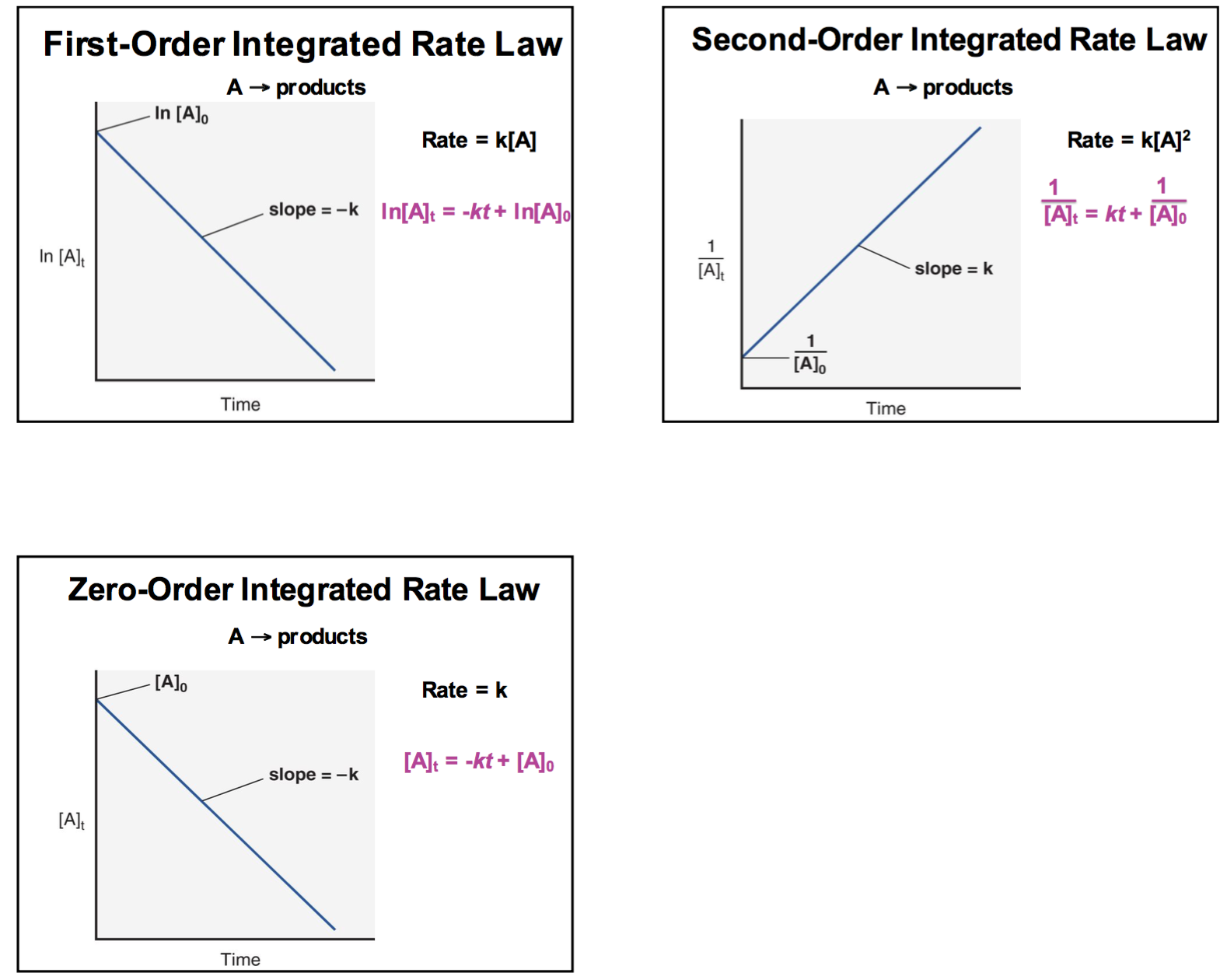

- Sun Mar 03, 2019 11:17 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Does anyone know if test 2 will be curved?
- Replies: 15
- Views: 3014
Re: Does anyone know if test 2 will be curved?
Here is what Lavelle said in the syllabus: "Each test and exam has a total score but is not assigned a grade. Only at the end of the class when the class average score (out of 500 points) is known are final grades assigned. This class does not use a curve." So, its probably not very likely...
- Sun Mar 03, 2019 11:12 pm
- Forum: General Rate Laws
- Topic: Difference between First Order Reactions and Second Order Reactions
- Replies: 1
- Views: 493
Re: Difference between First Order Reactions and Second Order Reactions
https://chem.libretexts.org/Bookshelves/Physical_and_Theoretical_Chemistry_Textbook_Maps/Supplemental_Modules_(Physical_and_Theoretical_Chemistry)/Kinetics/Experimental_Methods/Methods_of_Determining_Reaction_Order has some useful descriptions to help differentiate them: "In a first-order reac...
- Tue Feb 26, 2019 4:37 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Platinum in cell diagrams
- Replies: 4
- Views: 1514
Re: Platinum in cell diagrams
This makes sense, but I'm confused, because for the answer of 6L.5 part b in the 7th edition (which i listed below), despite Iodine being a solid, platinum is still included? In the solution manual, it does say that "An inert electrode such as Pt is necessary when both oxidized and reduced spec...
- Tue Feb 26, 2019 12:09 am
- Forum: Work, Gibbs Free Energy, Cell (Redox) Potentials
- Topic: 6L.3 7th Edition: Solve for E° values?
- Replies: 1
- Views: 268
6L.3 7th Edition: Solve for E° values?
For problem 6L.3 in the 7th edition, how are we supposed to find the E° values of the cathode and anode? Is there an index in the back of the book that we are supposed to look the values up in, or do we just solve for them using an equation? In addition, if we do have to solve for them, what equatio...
- Sun Feb 24, 2019 7:57 pm
- Forum: Balancing Redox Reactions
- Topic: Oxidation and Reduction?
- Replies: 6
- Views: 872
Re: Oxidation and Reduction?
In a redox reaction, oxidation involves a loss of electrons, and reduction involves a gain of electrons. Some mnemonic devices that might be helpful include: LEO the lion says GER, where LEO = Loss of Electrons is Oxidation and GER = Gain of Electrons is Reduction OIL RIG, where OIL = Oxidation Is L...
- Sun Feb 24, 2019 7:49 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Gibbs Free Energy
- Replies: 7
- Views: 921
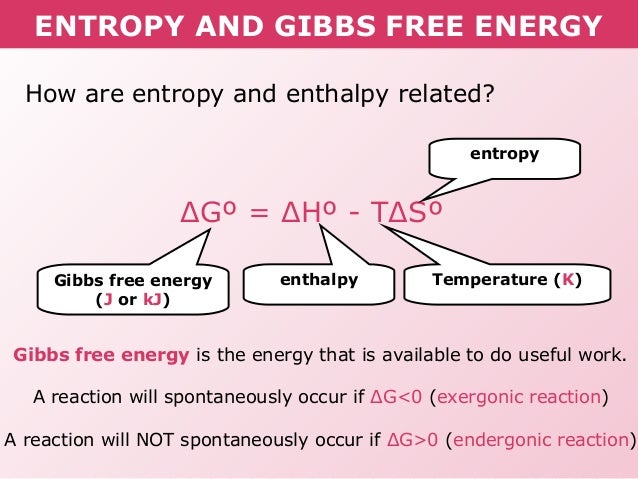
Re: Gibbs Free Energy
http://image.slidesharecdn.com/tang05-entropyandgibbsfreeenergy-120209095951-phpapp01/95/tang-05-entropy-and-gibbs-free-energy-13-638.jpg?cb=1422605471 What do exergonic and endergonic mean? In a reaction, exergonic means that energy is released to the surroundings, and endergonic means that energy...
- Sun Feb 24, 2019 7:46 pm
- Forum: Work, Gibbs Free Energy, Cell (Redox) Potentials
- Topic: Spontaneous?
- Replies: 6
- Views: 765
Re: Spontaneous?
Here is a table that might help:


- Sun Feb 24, 2019 7:44 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Gibbs free energy units
- Replies: 3
- Views: 1782
Re: Gibbs free energy units
Gibbs free energy is measured in units of kJ/mol
- Sun Feb 24, 2019 7:34 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Endothermic Reactions Spontaneous
- Replies: 2
- Views: 361
Re: Endothermic Reactions Spontaneous
Here is a table which might help:


- Sun Feb 17, 2019 9:34 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Gibbs Free Energy
- Replies: 3
- Views: 398
- Sun Feb 17, 2019 9:33 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Gibbs Free Energy
- Replies: 7
- Views: 921
- Sun Feb 17, 2019 9:28 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Nonexpansion Work
- Replies: 2
- Views: 375
Re: Nonexpansion Work
viewtopic.php?t=9861
In summary, "Expansion work is pressure-volume work, and nonexpansion work is anything else (electrical, friction, motional, etc.)."
In summary, "Expansion work is pressure-volume work, and nonexpansion work is anything else (electrical, friction, motional, etc.)."
- Sun Feb 17, 2019 8:56 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: delta G
- Replies: 3
- Views: 430
- Tue Feb 12, 2019 1:35 am
- Forum: Entropy Changes Due to Changes in Volume and Temperature
- Topic: Homework Problem 4f.7 7th edition
- Replies: 1
- Views: 247
Homework Problem 4f.7 7th edition
I'm confused on 4F.7 a, because I keep getting 6.65 J*K^-1 rather than 6.80 J*K^-1. Is this most likely due to rounding differences? If so, if anyone got 6.80 J*K^-1, can you mention what values you rounded to? Oh, and for reference, here is what my problem looks like, which also lines up with the s...
- Sun Feb 10, 2019 2:43 pm
- Forum: General Science Questions
- Topic: Thermodynamics in the Work Field
- Replies: 2
- Views: 601
Thermodynamics in the Work Field
I'm just curious, what are some common applications of thermodynamics in the work field? In addition, are there any specific examples of thermodynamics in relation to life sciences?
Edit: To clarify, I meant "work" in reference to occupation, not work in the thermodynamical sense.
Edit: To clarify, I meant "work" in reference to occupation, not work in the thermodynamical sense.
- Sun Feb 10, 2019 2:35 pm
- Forum: Student Social/Study Group
- Topic: Post All Chemistry Jokes Here
- Replies: 9651
- Views: 3591469
Re: Post All Chemistry Jokes Here
Honestly, it's kinda hard to come up with clever jokes for this thread. All the good ones argon.
- Sun Feb 10, 2019 2:30 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Using Bond Enthalpies
- Replies: 2
- Views: 321
Re: Using Bond Enthalpies
In addition, here is a chart with some common average bond enthalpies if you need it:
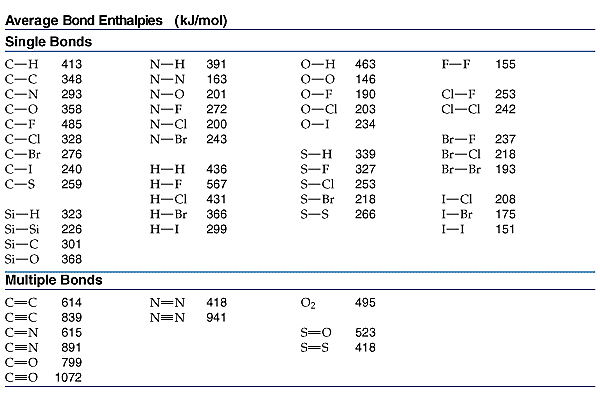

- Sun Feb 10, 2019 2:27 pm
- Forum: Calculating Work of Expansion
- Topic: Integrals
- Replies: 3
- Views: 364
Re: Integrals
No, I don't believe so.
Some further comments about this equation were made in the topic below:
viewtopic.php?f=129&t=41797&hilit=integral
Some further comments about this equation were made in the topic below:
viewtopic.php?f=129&t=41797&hilit=integral
- Sun Feb 10, 2019 2:23 pm
- Forum: Phase Changes & Related Calculations
- Topic: Enthalpy values
- Replies: 3
- Views: 518
Re: Enthalpy values
In addition, here is a chart with specific values pertaining to water:
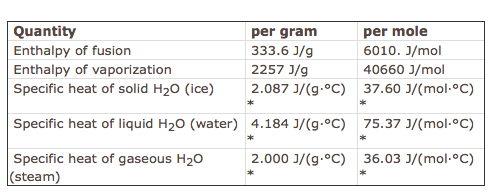

- Fri Feb 01, 2019 1:52 pm
- Forum: Thermodynamic Systems (Open, Closed, Isolated)
- Topic: Isolated Systems
- Replies: 6
- Views: 781
Isolated Systems
Regarding Isolated systems (other than the example of the entire universe, which Lavelle mentioned in class), is there any way we can prove that other so-called isolated systems are actually 100% isolated?
- Fri Feb 01, 2019 1:41 pm
- Forum: Phase Changes & Related Calculations
- Topic: PΔV of Solids and Liquids
- Replies: 4
- Views: 472
PΔV of Solids and Liquids
I understand that when solids and liquids are concerned in relation to the reaction constant P, PΔV is considered insignificant, but why is a change in volume ignored in cases involving substances like water, which visibly increases in volume when frozen?
- Wed Jan 30, 2019 1:36 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Exothermic vs. Endothermic
- Replies: 10
- Views: 3188
- Wed Jan 30, 2019 1:31 pm
- Forum: Phase Changes & Related Calculations
- Topic: Water phase change graph
- Replies: 6
- Views: 1478
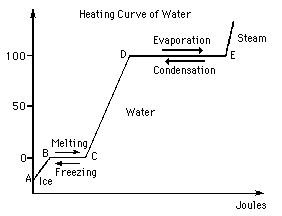
Re: Water phase change graph
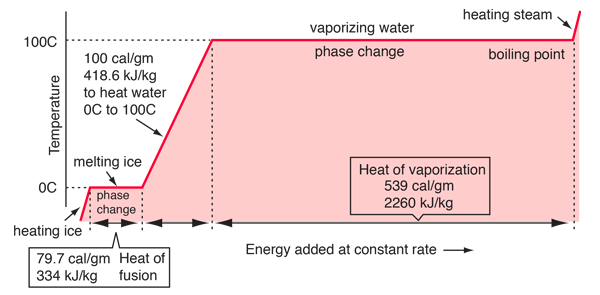
In this graph, the straight lines represent phase changes (where temperature is constant), and the slanted lines represent an increase in temperature.
- Wed Jan 30, 2019 1:28 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: Lecture
- Replies: 5
- Views: 495
- Wed Jan 30, 2019 1:22 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Standard State
- Replies: 3
- Views: 325
Re: Standard State
In addition, when pure elements are in their standard states, their standard enthalpies of formation are equal to zero, which makes solving for the enthalpy of a reaction easier.
- Wed Jan 30, 2019 1:15 pm
- Forum: Thermodynamic Definitions (isochoric/isometric, isothermal, isobaric)
- Topic: Heat vs. Temp
- Replies: 9
- Views: 930
Re: Heat vs. Temp
Temperature indicates the random motion of particles.
Heat is the transfer of energy due to a temperature difference.
In summary, Temperature represents particle motion, and Heat represents energy transfer.
Heat is the transfer of energy due to a temperature difference.
In summary, Temperature represents particle motion, and Heat represents energy transfer.
- Sun Jan 27, 2019 10:00 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Enthalpy
- Replies: 1
- Views: 304
Re: Enthalpy
Yes, it does apply to both forward and reverse reactions.
This forum post helps to flesh out why enthalpies vary depending of the method of calculation you use:
https://chemistry.stackexchange.com/que ... n-the-calc
This forum post helps to flesh out why enthalpies vary depending of the method of calculation you use:
https://chemistry.stackexchange.com/que ... n-the-calc
- Fri Jan 25, 2019 1:57 am
- Forum: Phase Changes & Related Calculations
- Topic: State functions
- Replies: 4
- Views: 1030
Re: State functions
In addition, here is an article about the difference between heat and temperature, and why they're not interchangeable: https://www.khanacademy.org/science/chemistry/thermodynamics-chemistry/internal-energy-sal/a/heat In addition, http://www.physicstutorials.org/home/heat-temperature-and-thermal-exp...
- Fri Jan 25, 2019 1:53 am
- Forum: Phase Changes & Related Calculations
- Topic: State functions
- Replies: 4
- Views: 1030
Re: State functions
According to http://chemed.chem.purdue.edu/genchem/topicreview/bp/ch5/work.php: "Heat and work are not state functions. Work can't be a state function because it is proportional to the distance an object is moved, which depends on the path used to go from the initial to the final state. If work...
- Fri Jan 25, 2019 1:41 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Units When Calculating Equilibrium Constant
- Replies: 4
- Views: 480
Re: Units When Calculating Equilibrium Constant
In addition, since K is equal to mol*L^-1 divided by mol*L^-1, the units will cancel out, leaving a unitless constant K.
- Mon Jan 21, 2019 1:36 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Strong acids and bases
- Replies: 4
- Views: 488
Re: Strong acids and bases
Yeah, it is probably best to just memorize the list of the 7 strong acids and 8 strong bases.
Here is a link to a list if you need it: http://www.austincc.edu/chemlab/strongacidbase
Here is a link to a list if you need it: http://www.austincc.edu/chemlab/strongacidbase
- Mon Jan 21, 2019 1:26 pm
- Forum: Bronsted Acids & Bases
- Topic: Sig Figs
- Replies: 4
- Views: 505
Re: Sig Figs
I doubt you would get a whole lot of points taken off, but I know Professor Lavelle posted a resource on the class website going over sig fig rules if you want to brush up on them.
- Sun Jan 13, 2019 6:38 pm
- Forum: Ideal Gases
- Topic: Units
- Replies: 3
- Views: 317
Re: Units
According to google... torr = "a unit of pressure used in measuring partial vacuums, equal to 133.32 pascals" bar = "a unit of pressure equivalent to 100,000 newtons per square meter or approximately one atmosphere" atm = "a unit of pressure defined as 101,325 Pa" pasca...
- Fri Jan 11, 2019 4:27 pm
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Inert Gases
- Replies: 2
- Views: 172
Re: Inert Gases
Actually, I don't think that an inert gas affects the equilibrium constant at all because if the pressure of a reaction vessel is increased by adding an inert gas, the moles of reactant/product and the volume remain constant. Essentially, unless there is a change in reactant/product concentration, t...
- Thu Jan 10, 2019 6:28 pm
- Forum: Ideal Gases
- Topic: HW # 5G.5 7th edition
- Replies: 2
- Views: 213
Re: HW # 5G.5 7th edition
If you look at page three of the syllabus, you will notice that 5G.5 is not included in the list of homework problems.
- Thu Jan 10, 2019 6:24 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: 5G.5
- Replies: 2
- Views: 207
Re: 5G.5
If you look at page three of the syllabus, you will notice that 5G.5 is not included in the list of homework problems.
- Thu Jan 10, 2019 6:21 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Pure solids and liquids in eq. constants [ENDORSED]
- Replies: 4
- Views: 1940
Re: Pure solids and liquids in eq. constants [ENDORSED]
Pure solids and liquids are not included in equilibrium constants because the molar concentration of those substances does not change in reactions. In the case of aqueous solutions, H2O is not included in the constant equation because the overall change in the solvent concentration is insignificant ...