Search found 66 matches
- Wed Mar 13, 2019 12:00 am
- Forum: Arrhenius Equation, Activation Energies, Catalysts
- Topic: arrhenius equation
- Replies: 1
- Views: 308
Re: arrhenius equation
I don't think it was taught to use in lecture, but it might be tomorrow! My ta went over it in discussion, so I'm guessing it will still be good to know and understand it!
- Tue Mar 12, 2019 11:58 pm
- Forum: General Rate Laws
- Topic: rate laws and graphs
- Replies: 4
- Views: 553
Re: rate laws and graphs
I think it is important to understand the graphs. I doubt we would need to draw them, but knowing the what the slopes are for different orders is probably useful. I think some practice problems even incorporate the graphs, and it might be hard to solve them if you do not know what the axes/slope wou...
- Mon Mar 11, 2019 2:54 pm
- Forum: Reaction Mechanisms, Reaction Profiles
- Topic: Frequency Factor
- Replies: 5
- Views: 547
Re: Frequency Factor
I think frequency factor accounts for the frequency in which two compounds (reactants) will collide with each other at the correct orientation. As mentioned, in class when the frequency factor increases, k will also increase.
- Mon Mar 11, 2019 2:51 pm
- Forum: First Order Reactions
- Topic: Half-life Clarification
- Replies: 5
- Views: 617
Re: Half-life Clarification
Yes. Each rate law has its own equation for 1/2 life. For example, the half-life for the 1st order is 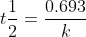 and the half-life for 2nd order is
and the half-life for 2nd order is 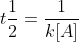 .
.
- Thu Mar 07, 2019 10:10 am
- Forum: Work, Gibbs Free Energy, Cell (Redox) Potentials
- Topic: Test 2 Gibbs Free Energy
- Replies: 5
- Views: 708
Re: Test 2 Gibbs Free Energy
Ice melting is a spontaneous reaction and when you have a spontaneous reaction,  G is automatically negative. If it was a non-spontaneous reaction
G is automatically negative. If it was a non-spontaneous reaction  G would be positive.
G would be positive.
- Wed Mar 06, 2019 3:59 pm
- Forum: First Order Reactions
- Topic: 1/2 life
- Replies: 7
- Views: 802
Re: 1/2 life
1/2 life for first order is the only order that is not dependent on initial concentration but just the reaction constant (k), with units 1/s.
- Tue Mar 05, 2019 3:34 pm
- Forum: First Order Reactions
- Topic: First order graph
- Replies: 7
- Views: 871
Re: First order graph
It represents time and it is usually measured in seconds, therefore rate = M/s, but it can also be measured in minutes, hours... depending on the reaction.
- Sun Mar 03, 2019 4:43 pm
- Forum: Balancing Redox Reactions
- Topic: Oxidation number?
- Replies: 12
- Views: 7528
Re: Oxidation number?
There are some rules that are helpful to identify an elements oxidations number, based on there location in the periodic table. For example, if the element is in group 1, its oxidation number is always +1. Likewise, if it is in group 2, the oxidation number is always +2.
- Sun Mar 03, 2019 4:39 pm
- Forum: Kinetics vs. Thermodynamics Controlling a Reaction
- Topic: Electrolysis
- Replies: 4
- Views: 536
Re: Electrolysis
Electrolysis uses an electric current, from an outside source, in order to produce a non-spontaneous reaction (where  G > 0)
G > 0)
- Sun Mar 03, 2019 4:31 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Test 2 Scores
- Replies: 6
- Views: 610
Re: Test 2 Scores
I think we should be getting them back this week in discussions sections.
- Sun Mar 03, 2019 4:30 pm
- Forum: Kinetics vs. Thermodynamics Controlling a Reaction
- Topic: Battery
- Replies: 2
- Views: 384
Re: Battery
They are not the same things, but they are similar as a rechargeable battery can act as an electrolytic cell when they are being recharged.
- Thu Feb 21, 2019 11:08 pm
- Forum: Van't Hoff Equation
- Topic: Practice Problems
- Replies: 3
- Views: 536
Re: Practice Problems
I don’t think you would specifically need to use the Van Hoff’s relationship, as long as you know the relationship conceptually.
- Thu Feb 21, 2019 10:39 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Gibbs Free Energy in cal
- Replies: 2
- Views: 291
Re: Gibbs Free Energy in cal
It should be measured in cal/mol or J/mol. This conversion is 1 J= 0.239 cal but you probably won't need to convert, just use which ever is given.
- Thu Feb 21, 2019 10:35 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Delta G = 0
- Replies: 4
- Views: 428
Re: Delta G = 0
I believe that if  G is equal to zero the reaction is at equilibrium.
G is equal to zero the reaction is at equilibrium.
- Tue Feb 19, 2019 4:48 pm
- Forum: Calculating Standard Reaction Entropies (e.g. , Using Standard Molar Entropies)
- Topic: Midterm Grades
- Replies: 35
- Views: 2777
Re: Midterm Grades
Ethan Breaux 2F wrote:are there ever curves?
I don't think there are ever curves on the tests/midterm.
- Wed Feb 13, 2019 11:57 am
- Forum: Third Law of Thermodynamics (For a Unique Ground State (W=1): S -> 0 as T -> 0) and Calculations Using Boltzmann Equation for Entropy
- Topic: Thermodynamic Laws
- Replies: 1
- Views: 430
Re: Thermodynamic Laws
I would be prepared to understand/explain them conceptually as well as through calculations.
- Wed Feb 13, 2019 11:52 am
- Forum: Phase Changes & Related Calculations
- Topic: Isothermal
- Replies: 9
- Views: 1146
Re: Isothermal
I think it will be stated in the questions, but if it doesn't specifically say isothermal, it would say that temperature remains constant.
- Wed Feb 13, 2019 11:48 am
- Forum: Thermodynamic Definitions (isochoric/isometric, isothermal, isobaric)
- Topic: Constant pressure
- Replies: 1
- Views: 519
Constant pressure
I know that when there is constant temperature  U = 0, but if there is constant pressure in a system, what things can we say about it?
U = 0, but if there is constant pressure in a system, what things can we say about it?
- Tue Feb 05, 2019 8:10 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: Equipartition Theorem
- Replies: 1
- Views: 301
Re: Equipartition Theorem
The Equipartition Theorem is not included on the third outline: Thermochemistry and The First Law of Thermodynamics, so I do not think we will need to know it.
- Tue Feb 05, 2019 8:05 pm
- Forum: Thermodynamic Definitions (isochoric/isometric, isothermal, isobaric)
- Topic: Ideal Gas
- Replies: 3
- Views: 417
Re: Ideal Gas
I do not believe there is like a specific ideal gas, it is just a theoretical way to describe the gas. It means that it obeys the gas laws and therefore easier to analyze.
- Tue Feb 05, 2019 8:03 pm
- Forum: Entropy Changes Due to Changes in Volume and Temperature
- Topic: Change in Entropy at a Constant Heat Capacity
- Replies: 1
- Views: 199
Re: Change in Entropy at a Constant Heat Capacity
I think because the heat capacity is constant the value does not need to be included when solving a problem, therefore you can use the equation they give you for  S in order to find entropy, and use the values that should be provided for moles, and the two temperatures.
S in order to find entropy, and use the values that should be provided for moles, and the two temperatures.
- Wed Jan 30, 2019 2:24 pm
- Forum: Phase Changes & Related Calculations
- Topic: Work
- Replies: 5
- Views: 553
Re: Work
Work is more complex than a state property. Work is dependent on the path used to get to its current state, which is the opposite of the definition of a state property which only depends on the state of the system.
- Wed Jan 30, 2019 2:20 pm
- Forum: Phase Changes & Related Calculations
- Topic: J vs. kJ
- Replies: 9
- Views: 1090
Re: J vs. kJ
I think problems will normally specify what ids preferred or if they give you a value with J or kJ included, use the one that is given. Otherwise you should be able to do either and just make sure the units are present.
- Wed Jan 30, 2019 2:18 pm
- Forum: Calculating Work of Expansion
- Topic: Units for Work
- Replies: 6
- Views: 711
Re: Units for Work
I think for pressure we use atm and for volume it should be Liters/milliliters, depending on the problem provides.
- Wed Jan 23, 2019 7:34 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Enthalpy and q
- Replies: 3
- Views: 319
Re: Enthalpy and q
Dr. Lavelle said that a state property is a value determined by its current state, independent of the path taken to obtain that state. Enthalpy does fall under this category, along with temperatures, volume, pressure, energy, density and heat capacity.
- Wed Jan 23, 2019 7:09 pm
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Strong Acids and Bases
- Replies: 5
- Views: 516
Re: Strong Acids and Bases
I don't think you need to have any particular compounds memorized as strong acids or bases, but it would probably be good to know a couple (like some that show up frequently in lectures/homework problems). More importantly, just know how to identify compounds as acids/bases.
- Wed Jan 23, 2019 7:03 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Calculating pH of Weak Acids and Bases
- Replies: 2
- Views: 302
Re: Calculating pH of Weak Acids and Bases
I think that is totally correct, and Dr. Lavelle mentioned the metaphor that if a person has a million dollars giving away a couple thousand doesn't make much of a difference to them, because they are still very close to a million. So basically even though we don't know x, we know it is going to be ...
- Sat Jan 19, 2019 3:56 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: temperature
- Replies: 2
- Views: 195
Re: temperature
I think that if you increase the temperature the rate of the reaction increases, but I do not believe it affects concentration or pressure in any way.
- Thu Jan 17, 2019 4:31 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Conjugate base
- Replies: 7
- Views: 729
Conjugate base
How can you tell if something is a conjugate base? Any info about conjugate bases would be helpful!
- Wed Jan 16, 2019 4:23 pm
- Forum: Non-Equilibrium Conditions & The Reaction Quotient
- Topic: relationship between Ka, Kb, and its ability to donate
- Replies: 3
- Views: 277
Re: relationship between Ka, Kb, and its ability to donate
That sounds right! Also, as Ka increases, Kb decreases and vice versa, since Ka x Kb = Kw.
- Sat Jan 12, 2019 10:03 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Solving for K
- Replies: 7
- Views: 474
Re: Solving for K
I think that as long as you know that the brackets represent concentration you should be good. Once you begin to solve the problem, I don't think it is necessary to differentiate between parentheses or brackets.
- Sat Jan 12, 2019 9:47 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: solids & liquids
- Replies: 5
- Views: 452
Re: solids & liquids
Aqueous solutions are also left out when finding K and these also apply when finding Q.
- Wed Jan 09, 2019 2:08 pm
- Forum: Non-Equilibrium Conditions & The Reaction Quotient
- Topic: ICE Tables
- Replies: 3
- Views: 614
Re: ICE Tables
I think the ICE table is just a way to organize the work and to go from Initial molarity(I) to Change in molarity(C) to the Equilibrium Molarity(E). I found this video helpful with explaining and showing an example: https://www.youtube.com/watch?v=iDr5DMiJ1j8
- Tue Jan 08, 2019 1:34 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: K'
- Replies: 3
- Views: 288
Re: K'
I think k' refers to the inverse of k, meaning if we know k of a forward reaction, the reverse reaction equals k' or 1/k.
- Sat Dec 08, 2018 6:44 pm
- Forum: Properties & Structures of Inorganic & Organic Bases
- Topic: Ethyl Alcohol vs Methyl Alcohol
- Replies: 1
- Views: 559
Re: Ethyl Alcohol vs Methyl Alcohol
Ethyl alcohol is a larger compound, as you can see in its formula, this means that the LDF/VDW forces that are acting on it are stronger than those forces present in the Methyl alcohol, therefore making it more difficult to dissociate, which is why the boiling point is higher.
- Thu Dec 06, 2018 9:35 pm
- Forum: Lewis Acids & Bases
- Topic: Strong Acids
- Replies: 6
- Views: 631
Re: Strong Acids
I think that thinking of this aside from the size of atoms, you can think about the electronegativity differences to determine bond strength.
- Thu Dec 06, 2018 9:30 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Blood pH
- Replies: 1
- Views: 191
Re: Blood pH
I don't think we will be tested on this information. I think Prof. Lavelle was just informing us that the textbook has more information on blood pH, as he didn't go into much depth on it.
- Thu Nov 29, 2018 11:37 am
- Forum: Lewis Acids & Bases
- Topic: HF
- Replies: 5
- Views: 593
Re: HF
If HF could easily ionize into a solution it would not be as weak, however it does not fully ionize because its bonds are too strongly bound.
- Thu Nov 29, 2018 11:31 am
- Forum: Bond Lengths & Energies
- Topic: Bond Lengths
- Replies: 1
- Views: 478
Re: Bond Lengths
Sulfur wants 6 valence electrons, so whether there are two or three oxygens attached, there will be double bonds attached to all the oxygens, and therefore the bond lengths will be the same in both molecules.
- Thu Nov 29, 2018 11:20 am
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Polar vs Non-Polar
- Replies: 2
- Views: 292
Re: Polar vs Non-Polar
When a compound has a high electronegativity it can be considered more ionic and bonds that have more ionic characteristics they can be considered polar. Oppositely, compounds are non-polar if they have have a low electronegativity, because the electrons share more equally.
- Tue Nov 20, 2018 11:17 am
- Forum: Hybridization
- Topic: d Orbital
- Replies: 6
- Views: 590
Re: d Orbital
I think as long as you can recognize them, and understand the concept of hybrid d-orbitals you should be good!
- Tue Nov 20, 2018 11:16 am
- Forum: Hybridization
- Topic: Bond Angles
- Replies: 4
- Views: 459
Re: Bond Angles
I think if you are given a structure name or you can determine the structure based on a chemical formula, you can also determine bond angles, you do not need to draw the molecular shape, but it can be helpful to visualize it.
- Mon Nov 19, 2018 9:36 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Importance of Molecular Shape
- Replies: 2
- Views: 371
Re: Importance of Molecular Shape
Molecular shape can help to determine the molecules properties. For example, water is a good solvent because of its bent structure and bond angle of 104.5.
- Sun Nov 18, 2018 10:58 pm
- Forum: Interionic and Intermolecular Forces (Ion-Ion, Ion-Dipole, Dipole-Dipole, Dipole-Induced Dipole, Dispersion/Induced Dipole-Induced Dipole/London Forces, Hydrogen Bonding)
- Topic: Interaction Potential Energy
- Replies: 3
- Views: 424
Re: Interaction Potential Energy
If something is highly electronegative, electrons from another ion will be pulled away, distorting the shape. A good example of this is XeF4, where the F molecules are highly electronegative and so the electrons will be pulled to the F.
- Sun Nov 18, 2018 10:52 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: lone pairs
- Replies: 11
- Views: 883
Re: lone pairs
One example that Dr. Lavelle gave in class was that H2O has a lower bond angle than methane (CH4) because we know that methane has a bond angle of 109.5 degrees, and H20 is smaller than that, but we do not need to know that it is exactly 104.5 degrees.
- Tue Nov 13, 2018 8:38 pm
- Forum: Lewis Structures
- Topic: Lewis Structure
- Replies: 3
- Views: 1061
Lewis Structure
In discussion, we had to draw the Lewis Structure of ClCN. Which looks like: Cl-C \equiv N (with 2 lone pairs on Cl and one lone pair on N). I was wondering if there was anyway this compound could be represented with a triple bond on the Cl instead of the N, like: Cl \equiv C-N . And if this is not ...
- Tue Nov 13, 2018 5:08 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Homework Week 8 [ENDORSED]
- Replies: 7
- Views: 2170
Re: Homework Week 8 [ENDORSED]
Will we be having discussion sections next week if they are Monday-Wednesday?
- Sat Nov 10, 2018 2:20 pm
- Forum: Ionic & Covalent Bonds
- Topic: localized e- def
- Replies: 2
- Views: 224
Re: localized e- def
Localized electrons remain close to an atom or in the same place, however delocalized electrons are shown in different places between different resonance structures.
- Sat Nov 10, 2018 2:15 pm
- Forum: Ionic & Covalent Bonds
- Topic: Hydrogen bonding
- Replies: 7
- Views: 1352
Re: Hydrogen bonding
Aside from the number of bonds (single, double, etc.), the overall length of a bond also determines how strong it is. When the bond length is larger, the bond is weaker because the atoms are further apart and therefore they can break apart easier. H2O has a bond length of about 1A and H2S has a bond...
- Sat Nov 10, 2018 2:07 pm
- Forum: Resonance Structures
- Topic: Drawing resonance structures
- Replies: 5
- Views: 741
Drawing resonance structures
When asked to draw resonance structures, is it necessary to draw all the possible structures or just a few?
- Sun Nov 04, 2018 5:13 pm
- Forum: Lewis Structures
- Topic: 12b from the GarBreadium worksheet
- Replies: 3
- Views: 346
Re: 12b from the GarBreadium worksheet
When there is a negative charge, it means that it is more negative than it would have been without the charge, this means that another electron has been added. You represent this by drawing another electron or dot when drawing the Lewis Structure. So when you add up all the valence electrons from th...
- Sun Nov 04, 2018 5:08 pm
- Forum: Lewis Structures
- Topic: Lewis Structure(s)
- Replies: 3
- Views: 231
Re: Lewis Structure(s)
I'm not sure if the directions will specify or not, but I would recommend writing them all out and drawing attention to the one with the lowest energy.
- Sun Nov 04, 2018 5:02 pm
- Forum: Quantum Numbers and The H-Atom
- Topic: Number of Electrons based on principle quantum number
- Replies: 2
- Views: 2044
Number of Electrons based on principle quantum number
In one of the review sessions, it was asked what the maximum number of electrons would be present when n=2 or when n=3. I thought about how if n=2 then l=1 or l=0, which corresponds to the p-block and s-block, respectively. This would mean that the maximum electrons would be 6 (in the p-block). Is t...
- Sat Nov 03, 2018 11:59 am
- Forum: Ionic & Covalent Bonds
- Topic: Electronegativity Difference
- Replies: 6
- Views: 676
Re: Electronegativity Difference
Lavelle also mentioned that we would not be asked a question that resulted in a value like 1.6. He will make it more clear whether it is >2 (ionic) or <1.5 (covalent).
- Sun Oct 28, 2018 7:36 pm
- Forum: Ionic & Covalent Bonds
- Topic: Exceptions to Octet
- Replies: 2
- Views: 245
Exceptions to Octet
Can someone explain the reason to why H, He, Li, and Be are the exceptions to the octet rule?
- Sun Oct 28, 2018 7:31 pm
- Forum: Ionic & Covalent Bonds
- Topic: bound atoms
- Replies: 8
- Views: 1250
Re: bound atoms
When atoms bind together, there is a release of energy. Therefore bound atoms have less energy than single atoms. This is also the reason that bound atoms are more stable, because of the lower energy.
- Sun Oct 28, 2018 1:12 pm
- Forum: Student Social/Study Group
- Topic: Post All Chemistry Jokes Here
- Replies: 9651
- Views: 3612432
Re: Post All Chemistry Jokes Here
Did you know protons have mass? I didn't even know they were Catholic!
- Sun Oct 21, 2018 4:51 pm
- Forum: Heisenberg Indeterminacy (Uncertainty) Equation
- Topic: Heisenberg Post Module Assessment #16
- Replies: 2
- Views: 1698
Re: Heisenberg Post Module Assessment #16
I believe the answer to this is C, because we can’t know both the position and the momentum precisely, and if we know one of them precisely, we cannot precisely know the other. Lavelle also stated that there is a limit on the accuracy to which the momentum & position of a particle can be known s...
- Sun Oct 21, 2018 4:43 pm
- Forum: Einstein Equation
- Topic: The symbol v?
- Replies: 16
- Views: 8854
Re: The symbol v?
I don't think they are ever interchangeable since v (nu) in E=hv for instance stands for frequency, but v represents velocity in an equation like KE=1/2mv^2. They are two different things and can't be looked at as interchangeable.
- Sun Oct 21, 2018 4:33 pm
- Forum: Wave Functions and s-, p-, d-, f- Orbitals
- Topic: Test Question
- Replies: 8
- Views: 744
Re: Test Question
I think Lavelle wants us to know what the orbitals would look like and understand the differences but I don’t think it is required, and he said he would not make us draw any orbitals.
- Sun Oct 14, 2018 9:00 pm
- Forum: Photoelectric Effect
- Topic: SI Unit for Work Function
- Replies: 4
- Views: 1786
Re: SI Unit for Work Function
Although kJ/mol is a valid unit for energy/material, or something like finding  G. But for I’d use Joules.
G. But for I’d use Joules.
- Sun Oct 14, 2018 8:43 pm
- Forum: Properties of Electrons
- Topic: Electron Question
- Replies: 6
- Views: 596
Re: Electron Question
I think what they mean is that electrons have a range of values instead of continuous energy levels, implying an unending range, which is why an electron can’t exist in every case but rather a specific or quantized level.
- Sun Oct 14, 2018 8:31 pm
- Forum: Properties of Light
- Topic: Measurable Wavelengths
- Replies: 3
- Views: 194
Re: Measurable Wavelengths
I believe in lecture, we were told that for the sake of answering questions that ask if there can be measurable wavelike properties the cutoff is at 10^-18 (even though it is extremely small), but in the lab, it would be rare that anyone measures pat 10^-12.
- Sun Oct 07, 2018 9:07 pm
- Forum: Properties of Light
- Topic: Question 1A3.
- Replies: 1
- Views: 110
Re: Question 1A3.
C states that the extent of the change in the electric field at a given point decreases. This is true when the frequency decreases because if frequency is decreasing, wavelength must increase based on the equation c= \lambda x v When wavelength increases the 'extent of change' aka the slope (of the ...
- Sun Oct 07, 2018 4:09 pm
- Forum: SI Units, Unit Conversions
- Topic: Modules?
- Replies: 8
- Views: 726
Re: Modules?
Some of the module videos also have an additional example or slightly different material than what we had for lectures, so they are not only helpful to go over examples we already saw but also a little bit of different information as well!
- Sun Oct 07, 2018 4:06 pm
- Forum: Limiting Reactant Calculations
- Topic: Module 3 Question
- Replies: 3
- Views: 223
Module 3 Question
Can the mass of a product (or products) be greater than the total mass of reactant (or reactants)?
I would like to say that the mass of the reactants=mass of the product so no it isn't possible, but an option for an answer is: “under some conditions”… is it ever possible??
I would like to say that the mass of the reactants=mass of the product so no it isn't possible, but an option for an answer is: “under some conditions”… is it ever possible??

