Search found 60 matches
- Thu Mar 14, 2019 11:30 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Calculating Equilibrium Composition
- Replies: 2
- Views: 361
Re: Calculating Equilibrium Composition
After you find the x values, you plug it into the ice table. Only one of the values will give an answer that makes sense because the other x value will give a negative equilibrium concentration, which is not possible.
- Thu Mar 14, 2019 11:27 am
- Forum: Method of Initial Rates (To Determine n and k)
- Topic: mmol vs mol
- Replies: 6
- Views: 1071
Re: mmol vs mol
If the problem does not specify what units your answer should be in then it doesn't matter which one you use.
- Thu Mar 14, 2019 11:23 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Equilibrium Partial pressure Units
- Replies: 2
- Views: 280
Re: Equilibrium Partial pressure Units
Partial pressure is usually given in either atm or bar.
- Sat Mar 09, 2019 7:29 pm
- Forum: Ideal Gases
- Topic: sre
- Replies: 5
- Views: 601
Re: sre
The standard reaction enthalpy is just the enthalpy when all reactants and products are in their standard state at 1 atm.
- Sat Mar 09, 2019 7:24 pm
- Forum: Reaction Mechanisms, Reaction Profiles
- Topic: catalyst/enzyme
- Replies: 4
- Views: 517
Re: catalyst/enzyme
A catalyst lowers the activation energy so it ends up speeding up the rate of the reaction.
- Sat Mar 09, 2019 7:18 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Negligible X
- Replies: 3
- Views: 394
Re: Negligible X
If the given K value is less than 10^-3 then you can use the approximation method where x is so small that the change it causes to the equilibrium concentration is negligible.
- Sun Mar 03, 2019 4:00 pm
- Forum: Balancing Redox Reactions
- Topic: Acidic vs basic solutions
- Replies: 10
- Views: 1043
Re: Acidic vs basic solutions
For acidic solutions you balance out the oxygens by adding H2O and you balance the hydrogens by adding H+. For basic solutions you balance the oxygens by adding H2O and then you balance the hydrogens by adding H2O to the side that requires more H atoms and add the same amount of OH- to the other side.
- Sun Mar 03, 2019 3:54 pm
- Forum: Van't Hoff Equation
- Topic: units of T
- Replies: 5
- Views: 848
Re: units of T
When doing calculations you should always convert temperature into kelvins. In general just keep track of the units at all times because some of the constants on the constants and equation sheets (such as specific heat capacity) are given in celsius so be mindful of that.
- Sun Mar 03, 2019 3:46 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: delta G
- Replies: 5
- Views: 719
Re: delta G
Delta G is the change in Gibbs free energy. The equation commonly used is: delta G = delta H - T(delta S). Gibbs free energy is also a state function.
- Sun Feb 24, 2019 12:03 pm
- Forum: Balancing Redox Reactions
- Topic: difference between oxidation and reduction
- Replies: 8
- Views: 994
Re: difference between oxidation and reduction
A loss of electrons is oxidation, a gain of electrons is reduction. If the charge increases then electrons were removed so it would be considered oxidation.
- Sun Feb 24, 2019 11:58 am
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Spontaneous cell reaction
- Replies: 2
- Views: 360
Re: Spontaneous cell reaction
A positive cell potential indicates the reaction is spontaneous as written for the given conditions.
- Sun Feb 24, 2019 11:28 am
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: Platinum
- Replies: 3
- Views: 414
Re: Platinum
The textbook states that you include an inert electrode such as platinum when the electrode reaction does not include a conducting solid as a reactant or product.
- Sun Feb 17, 2019 2:38 pm
- Forum: Entropy Changes Due to Changes in Volume and Temperature
- Topic: calculating delta S
- Replies: 4
- Views: 488
Re: calculating delta S
Pressure and volume are inversely proportional so (P1/P2) = (V2/V1).
- Sun Feb 17, 2019 2:37 pm
- Forum: Thermodynamic Systems (Open, Closed, Isolated)
- Topic: state functions and properties
- Replies: 11
- Views: 6035
Re: state functions and properties
A state function only depends current conditions, not the path it takes to get there.
- Sun Feb 17, 2019 2:33 pm
- Forum: Concepts & Calculations Using Second Law of Thermodynamics
- Topic: Heating Curve
- Replies: 3
- Views: 573
Re: Heating Curve
When there is a phase change you use the equation S = qrev/T because the temperature is constant. You use S = nClnT2/T1 when the temperature does change.
- Thu Feb 07, 2019 10:20 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: Work
- Replies: 3
- Views: 428
Re: Work
If work is done on the system, w is positive because the energy of the system has increased. If work is done by the system, w is negative because the energy of the system has decreased.
- Thu Feb 07, 2019 10:09 pm
- Forum: Thermodynamic Systems (Open, Closed, Isolated)
- Topic: Isolated System
- Replies: 4
- Views: 418
Re: Isolated System
In an isolated system, matter and energy are not exchanged with the surroundings. Therefore the internal energy remains constant, so deltaU is 0.
- Thu Feb 07, 2019 10:07 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Definitions of Heat Capacities
- Replies: 2
- Views: 363
Re: Definitions of Heat Capacities
Heat capacity is the heat required to raise the temperature of an object by 1 degree C.
Specific heat capacity is the heat required to raise 1g of substance by 1 degree C.
Molar heat capacity is heat required to raise 1 mol of substance by 1 degree C.
Specific heat capacity is the heat required to raise 1g of substance by 1 degree C.
Molar heat capacity is heat required to raise 1 mol of substance by 1 degree C.
- Thu Jan 31, 2019 10:30 am
- Forum: Thermodynamic Systems (Open, Closed, Isolated)
- Topic: Surroundings
- Replies: 11
- Views: 947
Re: Surroundings
The system is the object of interest and the surroundings is anything else, so there is no boundary to the surroundings. Essentially the system + surroundings = universe.
- Thu Jan 31, 2019 10:25 am
- Forum: Thermodynamic Systems (Open, Closed, Isolated)
- Topic: closed vs isolated
- Replies: 14
- Views: 1081
Re: closed vs isolated
In a closed system, energy can be exchanged with the surroundings. In an isolated system, neither matter nor energy can be exchanged with the surroundings.
- Thu Jan 31, 2019 10:21 am
- Forum: Phase Changes & Related Calculations
- Topic: Steam vs Boiling Water
- Replies: 10
- Views: 10307
Re: Steam vs Boiling Water
In the process of phase change (in this case vaporization) the temperature doesn't change (stays at 100C). However, when making steam, there is still heat and energy being added to break the bonds, which is why steam causes severe burns.
- Fri Jan 25, 2019 9:36 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: 5%
- Replies: 10
- Views: 808
Re: 5%
If you use the approximation method when using an ICE table, the x value you get needs to be less than 5% of the initial concentration for this method to be valid.
- Fri Jan 25, 2019 9:28 pm
- Forum: Phase Changes & Related Calculations
- Topic: Modules
- Replies: 17
- Views: 1328
Re: Modules
The modules are optional. The post assessments are pretty helpful however if you want extra practice.
- Fri Jan 25, 2019 9:26 pm
- Forum: Non-Equilibrium Conditions & The Reaction Quotient
- Topic: ICE table and Q
- Replies: 6
- Views: 1653
Re: ICE table and Q
You use ICE tables to find the concentrations at equilibrium because the whole point of an ICE table is to show the initial concentration, the change in concentration, and the equilibrium concentration. To find Q you just find the ratio of products to reactants at any point during the reaction.
- Thu Jan 17, 2019 10:29 pm
- Forum: Non-Equilibrium Conditions & The Reaction Quotient
- Topic: Reaction Quotient
- Replies: 10
- Views: 811
Re: Reaction Quotient
Both K and Q are calculated the same way. K just describes the reaction at equilibrium and tells us whether there are more products or reactants at equilibrium. Q can be calculated at any part of the reaction and is used to determine the direction which a reaction will proceed.
- Thu Jan 17, 2019 10:23 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Calculating PH of weak acids and bases
- Replies: 5
- Views: 392
Re: Calculating PH of weak acids and bases
Because the K value is so small you can use the approximation method. In this case the x value will be so small that you can assume the value (0.1-x) will be approximately equal to 0.1. You can use this method whenever K is less than 10^-3.
- Thu Jan 17, 2019 10:14 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Quadratic equation and ICE box
- Replies: 3
- Views: 304
Re: Quadratic equation and ICE box
If the K value is very small (less than 10^-3) then you can use the approximation method where you assume that x is so small it won't make a big difference. If the x value you get is less than 5% of the initial concentration, then using the approximation method is valid. Otherwise you need to use th...
- Fri Jan 11, 2019 6:23 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Charles’s Law
- Replies: 4
- Views: 224
Re: Charles’s Law
Charles' Law simply states that the volume of a gas is directly proportional to the temperature if pressure stays constant.
- Fri Jan 11, 2019 6:10 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Kp vs Kc
- Replies: 6
- Views: 627
Re: Kp vs Kc
Both are equally important. But keep in mind that Kp is only used for gases while Kc can be used for gases and aqueous solutions.
- Thu Jan 10, 2019 6:44 pm
- Forum: Ideal Gases
- Topic: Equilibrium Constant
- Replies: 3
- Views: 286
Re: Equilibrium Constant
The molar concentrations of solids and liquids do not change in a reaction and therefore are not used to calculate the equilibrium constant. On the other hand, both gases and aqueous solutions are used to calculate the equilibrium constant.
- Sat Dec 08, 2018 7:59 pm
- Forum: Octet Exceptions
- Topic: formal charge
- Replies: 2
- Views: 471
Re: formal charge
Formal charge is useful to find the most stable lewis structure. You want the formal charge to be 0 on all atoms but if that's not possible then usually the negative formal charge goes on the most electronegative atom.
- Sat Dec 08, 2018 7:51 pm
- Forum: Trends in The Periodic Table
- Topic: atomic radius
- Replies: 2
- Views: 595
Re: atomic radius
atomic radius is defined as half the distance between the centers of neighboring atoms
- Sat Dec 08, 2018 7:47 pm
- Forum: *Molecular Orbital Theory (Bond Order, Diamagnetism, Paramagnetism)
- Topic: types of bonds
- Replies: 14
- Views: 1227
Re: types of bonds
yes! a single bond has 1 sigma bond, a double bond has 1 sigma and 1 pi bond, and a triple bond has 1 sigma and 2 pi bonds.
- Sun Dec 02, 2018 5:05 pm
- Forum: Hybridization
- Topic: Hybridization
- Replies: 11
- Views: 1294
Re: Hybridization
A trigonal planar molecule has 3 regions of electron density. the regions of electron density is equal to the number of hybrid orbitals. Therefore a trigonal planar molecule has a hybridization of sp2 (a hybrid of 1 s orbital and 2 p orbitals).
- Sun Dec 02, 2018 4:57 pm
- Forum: Bronsted Acids & Bases
- Topic: Bronsted vs. Lewis
- Replies: 3
- Views: 501
Re: Bronsted vs. Lewis
A bronsted acid is a proton donor and a bronsted base is a proton acceptor. A lewis acid accepts an electron pair and a lewis base donates the electron pair. Both essentially mean the same thing however the lewis definition is a little more general. In the example: NH3 + H2O → NH4+ + OH- a hydrogen ...
- Sun Dec 02, 2018 4:35 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: VSEPR Model
- Replies: 5
- Views: 576
Re: VSEPR Model
when using the VSEPR model, you can essentially use the lewis structure to see how many lone and bonding pair electrons there are around the central atom to determine the AXE formula. From there you can describe shape, bond angles, polarity etc.
- Sun Nov 25, 2018 9:32 pm
- Forum: Bond Lengths & Energies
- Topic: Why Are Double Bonds Shorter
- Replies: 16
- Views: 6195
Re: Why Are Double Bonds Shorter
Its because the additional bonding electrons in a double bond attract the nuclei more strongly and pull the atoms closer together.
- Sun Nov 25, 2018 9:28 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: e- Density
- Replies: 4
- Views: 503
Re: e- Density
The electrons in double and triple bonds stay together and repel other bonds/lone pairs as a single unit so you treat it as just one region of electron density.
- Sun Nov 25, 2018 2:28 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Dipoles
- Replies: 2
- Views: 310
Re: Dipoles
When there is a difference between the electronegativity of two atoms a partial charge forms. The atom with more pulling power has a greater share of electrons so it has a negative partial charge which means it has a negative dipole.
- Thu Nov 15, 2018 6:05 pm
- Forum: SI Units, Unit Conversions
- Topic: VSEPR model
- Replies: 5
- Views: 3244
Re: VSEPR model
The VSEPR model accounts for bond angles and molecular shape. Regions of high electron concentration in a molecule repel one another so these regions move as far as possible to minimize repulsions.
- Thu Nov 15, 2018 5:57 pm
- Forum: Dipole Moments
- Topic: Dipoles
- Replies: 2
- Views: 176
Re: Dipoles
When there is a difference between the electronegativity of two atoms a partial charge forms. The atom with more pulling power has a greater share of electrons so it has a negative partial charge which means it has a negative dipole.
- Thu Nov 15, 2018 5:44 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: VSEPR model
- Replies: 3
- Views: 396
Re: VSEPR model
A multiple bond is just treated as one region of electron density. The electrons in double and triple bonds stay together and repel other bonds/lone pairs as a single unit.
- Sun Nov 11, 2018 1:24 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: trigonal pyramidal
- Replies: 3
- Views: 289
Re: trigonal pyramidal
In the trigonal pyramidal molecular shape there are three bonds and one lone pair on the central atom. This is the best shape because the lone pair on the central atom distorts the molecular shape to reduce lone pair-bonding pair repulsions. I'm sure this will be covered more in future lectures!
- Sun Nov 11, 2018 1:11 pm
- Forum: Interionic and Intermolecular Forces (Ion-Ion, Ion-Dipole, Dipole-Dipole, Dipole-Induced Dipole, Dispersion/Induced Dipole-Induced Dipole/London Forces, Hydrogen Bonding)
- Topic: Greater polarizability of larger molecules
- Replies: 6
- Views: 1156
Re: Greater polarizability of larger molecules
In larger atoms the outermost electrons are less tightly held because the nucleus exerts a weak control over these electrons. Therefore they are easily distorted.
- Sat Nov 10, 2018 4:42 pm
- Forum: Polarisability of Anions, The Polarizing Power of Cations
- Topic: Polarizability
- Replies: 4
- Views: 450
Re: Polarizability
In larger atoms, electrons are less tightly held because they are further from the nucleus which is why they are easily distorted
- Thu Nov 01, 2018 10:22 pm
- Forum: Properties of Light
- Topic: Midterm Question 5a
- Replies: 4
- Views: 494
Re: Midterm Question 5a
The electrons in the Hg atom have discrete energy values so they can only emit certain wavelengths of light.
- Thu Nov 01, 2018 10:17 pm
- Forum: Bond Lengths & Energies
- Topic: Lengths of Bonds
- Replies: 9
- Views: 661
Re: Lengths of Bonds
The book states that "multiple bonds are shorter than single bonds between the same two elements because the additional bonding electrons attract the nuclei more strongly and pull the atoms closer together" (7th ed. pg 100)
Hope this helps!
Hope this helps!
- Thu Nov 01, 2018 10:02 pm
- Forum: Lewis Structures
- Topic: Lewis Structure of Glycine
- Replies: 1
- Views: 218
Re: Lewis Structure of Glycine
It is one of the homework problems and I think anything that shows up on the homework is fair game for the test.
- Thu Oct 25, 2018 9:38 pm
- Forum: Properties of Light
- Topic: Electromagnetic Spectrum
- Replies: 5
- Views: 600
Re: Electromagnetic Spectrum
You do not need to fully memorize the electromagnetic spectrum but it may be helpful to know that visible red light is about 700nm and visible violet light is about 400nm because that was covered in lecture.
- Thu Oct 25, 2018 9:33 pm
- Forum: Quantum Numbers and The H-Atom
- Topic: Nodal Planes and test 2
- Replies: 3
- Views: 309
Re: Nodal Planes and test 2
Yes, know that the s orbital has no nodal planes, the p orbital has 1 nodal plane, and the d orbital has 2.
- Thu Oct 25, 2018 9:30 pm
- Forum: Wave Functions and s-, p-, d-, f- Orbitals
- Topic: Degeneracy
- Replies: 6
- Views: 582
Re: Degeneracy
The textbook says that if something is degenerate it has the same energy. For example, atomic orbitals that are in the same sub shell.
- Thu Oct 18, 2018 10:19 pm
- Forum: Wave Functions and s-, p-, d-, f- Orbitals
- Topic: Nodal Planes [ENDORSED]
- Replies: 4
- Views: 754
Re: Nodal Planes [ENDORSED]
Essentially in a nodal plane no electrons will be found. You should know how many nodal planes there are for each orbital (no nodal planes in s orbital, 1 in p orbital, simplest d orbital has 2, etc.)
- Thu Oct 18, 2018 9:55 pm
- Forum: Photoelectric Effect
- Topic: 1B.7
- Replies: 3
- Views: 188
Re: 1B.7
For part a you use the equation 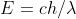
(because c and h are constants and the problem gives you the wavelength). This gives you the energy of a sodium atom. Then, for part b, after you find the number of atoms of sodium, you simply multiply it by the energy of one sodium atom.
(because c and h are constants and the problem gives you the wavelength). This gives you the energy of a sodium atom. Then, for part b, after you find the number of atoms of sodium, you simply multiply it by the energy of one sodium atom.
- Thu Oct 18, 2018 9:44 pm
- Forum: DeBroglie Equation
- Topic: De Broglie
- Replies: 12
- Views: 1464
Re: De Broglie
The DeBroglie equation only applies to particles with resting mass (for example electrons) that has momentum (p). You can't use this equation for something like light because light has no mass when at rest.
- Thu Oct 11, 2018 10:07 pm
- Forum: Properties of Light
- Topic: Problem 1A.9 (7th edition)
- Replies: 5
- Views: 294
Re: Problem 1A.9 (7th edition)
In the equation c=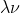 you do use meters (because the unit for c is meters per second)
you do use meters (because the unit for c is meters per second)
But it is important to note that wavelengths in the visible spectrum are usually given in nanometers (which is 10-9m) so just also know this conversion.
But it is important to note that wavelengths in the visible spectrum are usually given in nanometers (which is 10-9m) so just also know this conversion.
- Thu Oct 11, 2018 10:00 pm
- Forum: Photoelectric Effect
- Topic: Photoelectric Effect Equation
- Replies: 3
- Views: 365
Re: Photoelectric Effect Equation
Essentially each photon needs to have more energy than than the energy to remove an electron from the metal (aka the threshold energy). So if 1000 photons hit the metal surface and each individual photon has enough energy, then 1000 electrons will be released.
- Thu Oct 11, 2018 9:48 pm
- Forum: Properties of Light
- Topic: Calculate values of n
- Replies: 2
- Views: 183
Re: Calculate values of n
In this problem they give you the wavelength so you use the equation \nu =c/\lambda to find the frequency. Then you want to use the Rydberg equation which is \nu =R(1/n_{1}^{2}-1/n_{2}^{2}) . Your n 1 value will be 1 because it is in the uv spectrum so it is a part of the Lyman series. From ...
- Sat Oct 06, 2018 12:17 pm
- Forum: Empirical & Molecular Formulas
- Topic: HW F11
- Replies: 4
- Views: 480
Re: HW F11
NH4+ (ammonium) and H2PO4- (dihydrogen phosphate) are polyatomic ions which is why they wrote it like that.
- Thu Oct 04, 2018 5:51 pm
- Forum: Balancing Chemical Reactions
- Topic: 7th Edition L.35 question
- Replies: 3
- Views: 348
Re: 7th Edition L.35 question
I noticed that too and I believe the answer key is correct and the reactant should be Fe3Br8. This would make sense because the product from the second reaction should be one of the reactants in the 3rd reaction.
- Thu Oct 04, 2018 2:21 pm
- Forum: Accuracy, Precision, Mole, Other Definitions
- Topic: Polyatomic ions
- Replies: 3
- Views: 198
Re: Polyatomic ions
In the end you will have to memorize them but there are a few ways that will make memorizing them easier. 1. polyatomic ions ending with -ate have one more oxygen atom than those ending with -ite. (ex. nitrate: NO 3 - and nitrite: NO 2 - ) 2. ions with per- have one more oxygen atom then those endin...

