Search found 31 matches
- Sun Dec 09, 2018 9:42 am
- Forum: Conjugate Acids & Bases
- Topic: Stability of Conjugate Base
- Replies: 6
- Views: 3785
Re: Stability of Conjugate Base
A main reason for stability is attributed to resonance.
- Mon Dec 03, 2018 11:23 pm
- Forum: Naming
- Topic: Prefix bis, tris, tetrakis, etc. [ENDORSED]
- Replies: 5
- Views: 772
Re: Prefix bis, tris, tetrakis, etc. [ENDORSED]
An example of this - if you had (en)3 (ethylenediamine) for example, it would be tris-ethylenediamine.
- Mon Dec 03, 2018 11:19 pm
- Forum: Conjugate Acids & Bases
- Topic: Weak Acid, Strong Base
- Replies: 1
- Views: 305
Weak Acid, Strong Base
Why is it that the conjugate base of a weak acid is a strong base?
- Sun Dec 02, 2018 6:39 pm
- Forum: Amphoteric Compounds
- Topic: Examples
- Replies: 6
- Views: 620
Examples
What are some examples of amphoteric compounds other than water?
- Sun Dec 02, 2018 6:36 pm
- Forum: Naming
- Topic: Metal Prefixes
- Replies: 2
- Views: 305
Metal Prefixes
In example 17.29, Iron becomes ferro-. Do we need to know the names of metals used in coordination compounds? (such as argentate for silver, stannate for tin, etc.)
IUPAC
What does the New IUPAC Name Convention mean? It's on the reference sheet posted on Dr. Lavelle's website.
- Sun Nov 25, 2018 5:22 pm
- Forum: Hybridization
- Topic: Test 3
- Replies: 13
- Views: 914
Re: Test 3
We should probably be able to identify the shape and hybridization of a molecule, based on the ones Dr. Lavelle provided in class.
- Sun Nov 25, 2018 5:19 pm
- Forum: Bond Lengths & Energies
- Topic: Bonds in Water
- Replies: 5
- Views: 602
Re: Bonds in Water
Hydrogen bonds are involved in helping water molecules bond to other water molecules.
Covalent bonds are actually within the water molecule and bond the Hs and Os together.
Covalent bonds are actually within the water molecule and bond the Hs and Os together.
- Sun Nov 25, 2018 5:17 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Test 3
- Replies: 6
- Views: 575
Re: Test 3
Most likely yes, since we went over them in class.
- Sat Nov 17, 2018 3:32 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Formula for determining bond angle
- Replies: 6
- Views: 631
Re: Formula for determining bond angle
There isn't really a formula, I would just know the values of the main shapes. For example, know that trigonal planar is 120, tetrahedral is 109.5, and if there is a lone pair instead of a bond (such as in trigonal pyramidal), it would be slightly less than 109.5.
- Sat Nov 17, 2018 3:20 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Planar vs Pyramidal
- Replies: 5
- Views: 563
Re: Planar vs Pyramidal
With reference to trigonal planar, it has 3 bonds with no lone pairs. However, trigonal pyramidal has 3 bonds with one lone pair, thereby giving it a tetrahedral arrangement but a trigonal pyramidal shape. With regards to the bond angles, those in a trigonal planar would be 120, while those in a tri...
- Sat Nov 17, 2018 3:17 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Triple Bonds
- Replies: 2
- Views: 202
Triple Bonds
Why is it that triple bonds have 1 sigma and two pi bonds?
- Sat Nov 10, 2018 5:08 pm
- Forum: Resonance Structures
- Topic: Expanded Octet and Resonance
- Replies: 2
- Views: 400
Expanded Octet and Resonance
Can an atom without an expanded octet have resonance with more than 4 single bonds?
- Sat Nov 10, 2018 5:05 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Remembering VSEPR
- Replies: 2
- Views: 292
Remembering VSEPR
What’s the easiest way to remember the VSEPR shapes?
- Sat Nov 10, 2018 4:59 pm
- Forum: Ionic & Covalent Bonds
- Topic: Covalent Character
- Replies: 1
- Views: 256
Covalent Character
I’m still confused on how exactly covalent character works, is there a simple way to remember this? Thank you!
- Sat Nov 03, 2018 12:52 pm
- Forum: DeBroglie Equation
- Topic: HW 1.33b
- Replies: 2
- Views: 512
HW 1.33b
For homework problem 1.33, why is it that E = h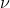 is used in part b as opposed to the work function? Isn't the energy required to release the electron the same as the threshold energy, or
is used in part b as opposed to the work function? Isn't the energy required to release the electron the same as the threshold energy, or 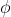 ?
?
- Fri Nov 02, 2018 11:00 am
- Forum: Electron Configurations for Multi-Electron Atoms
- Topic: Key Words
- Replies: 4
- Views: 505
Re: Key Words
Not necessarily keywords but great resources are The Organic Chemistry Tutor's video on YouTube, or Crash Course Chemistry. They go through all the key concepts for a particular subject and make it easier to remember.
- Fri Nov 02, 2018 10:58 am
- Forum: Electron Configurations for Multi-Electron Atoms
- Topic: Diamagnetic/Paramagnetic
- Replies: 1
- Views: 244
Diamagnetic/Paramagnetic
Hi,
In one of the review worksheets posted, there was a question on paramagnetic and diamagnetic electrons, do we need to know this for the midterm?
Also, should we know hybridization?
Thanks!
In one of the review worksheets posted, there was a question on paramagnetic and diamagnetic electrons, do we need to know this for the midterm?
Also, should we know hybridization?
Thanks!
- Thu Nov 01, 2018 12:17 pm
- Forum: Electron Configurations for Multi-Electron Atoms
- Topic: Losing Electrons
- Replies: 2
- Views: 304
Losing Electrons
When forming ions, do atoms first lose electrons from the 4p or 3d subshell? This is assuming 2 electrons have already been lost from the 4s subshell.
- Sat Oct 27, 2018 3:43 pm
- Forum: Quantum Numbers and The H-Atom
- Topic: Quantum Numbers
- Replies: 5
- Views: 517
Re: Quantum Numbers
You would have to draw out the orbital diagram. If the arrow is pointing up, it is +1/2 and if it is pointing down it is -1/2.
- Sat Oct 27, 2018 3:42 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Midterm
- Replies: 7
- Views: 764
Re: Midterm
It would probably just be on what we've done in class so far, unless stated otherwise.
- Sat Oct 27, 2018 3:41 pm
- Forum: Sigma & Pi Bonds
- Topic: determining which bonds are in a molecule
- Replies: 4
- Views: 512
Re: determining which bonds are in a molecule
Yes! You would have to write it out to find out the number of valence electrons, then you add them all up to figure out how many you need to place around your molecule.
- Sat Oct 20, 2018 8:24 pm
- Forum: Heisenberg Indeterminacy (Uncertainty) Equation
- Topic: Uncertainty
- Replies: 3
- Views: 416
Uncertainty
I'm having a bit of trouble understanding the uncertainty equation, why is it that the more certain you are about the momentum, the less certain you are about the position? Thank you!
- Sat Oct 20, 2018 8:20 pm
- Forum: Wave Functions and s-, p-, d-, f- Orbitals
- Topic: 4th quantum number question [ENDORSED]
- Replies: 3
- Views: 359
Re: 4th quantum number question [ENDORSED]
+1/2 means an electron is spinning up, while -1/2 means it is spinning down. These are the only two values that the fourth quantum number can be. An example: Electron configuration 2p3. If you draw the orbital diagram, you have one arrow up and one down in the first electron pair, and one arrow up i...
- Sat Oct 20, 2018 8:13 pm
- Forum: Properties of Electrons
- Topic: Balmer vs Lyman Series [ENDORSED]
- Replies: 5
- Views: 560
Re: Balmer vs Lyman Series [ENDORSED]
Lyman series occur in the UV spectrum. This means that the electron is going to the first energy level (n = 1). In a Balmer series, this is occurring in the visible light spectrum. So the electron will go to the second energy level, or n = 2. For this particular problem, you can assume that the nFin...
- Sun Oct 14, 2018 12:03 pm
- Forum: Properties of Light
- Topic: Speed of Light
- Replies: 41
- Views: 2834
Re: Speed of Light
You should treat it as a constant in any sort of calculations done for this class. I’m sure for higher level chemistry this would be different, but at this point I wouldn’t worry.
- Wed Oct 10, 2018 10:07 am
- Forum: Molarity, Solutions, Dilutions
- Topic: Finding the volume of stock solution to dilute
- Replies: 13
- Views: 1348
Re: Finding the volume of stock solution to dilute
Unless the problem specifically states that you need to keep the answer in L or convert it to mL, I wouldn't worry about it. However, since Molarity = moles/liters, it's a good idea to convert in the beginning itself.
- Wed Oct 10, 2018 9:52 am
- Forum: Photoelectric Effect
- Topic: Packets of Energy
- Replies: 2
- Views: 137
Packets of Energy
Can someone explain the concept of quanta being "packets of energy?" I'm having trouble visualizing this. (6th Edition page 11)
- Fri Oct 05, 2018 1:04 pm
- Forum: Properties of Light
- Topic: Behavior of small objects - clarification
- Replies: 3
- Views: 195
Behavior of small objects - clarification
Can someone explain what Dr. Lavelle meant by one discrete H2O molecule? I understand the concept of the water poured from the bucket, but the other part confused me.
- Wed Oct 03, 2018 11:55 pm
- Forum: Significant Figures
- Topic: Decimal point
- Replies: 14
- Views: 919
Decimal point
Can someone explain the difference in sig figs between 125 and 125. , if there is one?
- Wed Oct 03, 2018 11:23 pm
- Forum: Significant Figures
- Topic: Sig Figs... when to round?
- Replies: 7
- Views: 878
Re: Sig Figs... when to round?
To add on to what has already been said, it really helps if you store the value in your calculator as you are solving. That way, you get the most accurate answer. If you round as you go, your answer will probably be off by a few digits.

