Search found 93 matches
- Fri Mar 15, 2019 11:47 pm
- Forum: Work, Gibbs Free Energy, Cell (Redox) Potentials
- Topic: Cell Work
- Replies: 4
- Views: 664
Re: Cell Work
The maximum work done is related to the formula standard free energy = n*F*Ecell.
- Fri Mar 15, 2019 11:46 pm
- Forum: First Order Reactions
- Topic: pseudo first order
- Replies: 1
- Views: 515
Re: pseudo first order
Pseudo-first order is when you have a rate law with some number of reactants, such that we make all except for 1 reactant constant (usually by making them in large excess so that any reactant used in reaction is negligible) and manipulate the reaction from there.
- Fri Mar 15, 2019 11:28 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Standard Cell Potential
- Replies: 1
- Views: 235
Re: Standard Cell Potential
Standard cell potential is defined as cathode minus anode by convention.
- Mon Mar 11, 2019 9:04 pm
- Forum: Ideal Gases
- Topic: Ideal Gas Law as an Approximation
- Replies: 10
- Views: 996
Re: Ideal Gas Law as an Approximation
Typically, we can approximate and use the ideal gas law wherever there is a simple atomic or diatomic gas at STP like that of helium and nitrogen; these are the most like point-particles.
- Sat Mar 09, 2019 1:06 pm
- Forum: Reaction Mechanisms, Reaction Profiles
- Topic: Fast Step vs Slow Step
- Replies: 3
- Views: 387
Re: Fast Step vs Slow Step
Typically, we can determine which step to be the slow step by first looking at experimental analysis and roughly determining what the rate is proportional to. It is from this point that we have to "guess" the elementary steps and identify a step that has reactants that are found in our exp...
- Tue Mar 05, 2019 3:46 pm
- Forum: First Order Reactions
- Topic: Radioactive Decay
- Replies: 3
- Views: 366
Re: Radioactive Decay
It was through experimental verification that we were able to tell over a given period of time, now called a radioactive substance's half life, that half of the substance would decay away; this fits within our model of a first order process.
- Fri Mar 01, 2019 10:08 pm
- Forum: Method of Initial Rates (To Determine n and k)
- Topic: Units
- Replies: 6
- Views: 729
Re: Units
The units of the rate law vary with the order of the reaction, with the units of the general differential rate law given as moles/second ([change in concentration]/[change in time]).
- Fri Mar 01, 2019 8:55 am
- Forum: Balancing Redox Reactions
- Topic: Metals
- Replies: 2
- Views: 324
Re: Metals
Metals pack together in lattices that have delocalized electrons amongst positively charged nuclei, resembling a "sea of electrons". This phenomenon allows for conductance of electrons and thus current.
- Wed Feb 27, 2019 6:43 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Spontaneity [ENDORSED]
- Replies: 3
- Views: 466
Re: Spontaneity [ENDORSED]
Typically, if only considering entropy of a reaction, if delta S is positive, then the reaction is considered spontaneous. However, there are more cases where temperature and enthalpy would need to be considered (Gibbs free energy equation).
- Tue Feb 26, 2019 3:45 pm
- Forum: Balancing Redox Reactions
- Topic: common charges
- Replies: 2
- Views: 282
Re: common charges
Common oxidation states to note are that the alkali metals are typically +1, alkali earth metals are typically +2, oxygen is typically -2 (except for in peroxides, which would be -1), hydrogen is typically +1, fluorine is typically -1, and chlorine is typically -1.
- Tue Feb 26, 2019 3:41 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Potential Diff of Electrodes
- Replies: 1
- Views: 213
Re: Potential Diff of Electrodes
This is just the given convention, because of how we define cell diagrams with anodes on the left and cathodes on the right. Further discussion would result in a physical, rather than chemical, explanation of current and electron flow.
- Fri Feb 22, 2019 4:57 pm
- Forum: Concepts & Calculations Using Second Law of Thermodynamics
- Topic: Phase changes and Entropy
- Replies: 4
- Views: 670
Re: Phase changes and Entropy
Entropy is proportional to the amount of possible microstates possible, and when a liquid goes to the gaseous phase, it has more potential states to occupy.
- Fri Feb 22, 2019 4:42 pm
- Forum: Balancing Redox Reactions
- Topic: Moles of Charges, Adams, Disc 1a
- Replies: 2
- Views: 298
Re: Moles of Charges, Adams, Disc 1a
Basically treat n as how you have treated it thus far with balanced chemical reactions, now just also being mindful of Faraday's constant.
- Fri Feb 22, 2019 4:40 pm
- Forum: Balancing Redox Reactions
- Topic: Redox Reactions on Test 2
- Replies: 1
- Views: 217
Re: Redox Reactions on Test 2
Most likely we would be only need to worry about batteries in the upcoming test but still knowing how to balance redox reactions would be good in general, like in the case of acid/base reactions.
- Thu Feb 21, 2019 11:16 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Anode and Cathode
- Replies: 4
- Views: 489
Re: Anode and Cathode
The anode is considered to be your electron source and the cathode is considered to be your electron reservoir.
- Wed Feb 20, 2019 2:23 pm
- Forum: Balancing Redox Reactions
- Topic: Galvanic cells
- Replies: 4
- Views: 432
Re: Galvanic cells
Gradually, there will become an excess of ions built up on either side of the battery cell, which will impede current flow and stop the reaction.
- Fri Feb 15, 2019 1:07 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: enthalpy, entropy, Gibbs Free Energy signs
- Replies: 3
- Views: 357
Re: enthalpy, entropy, Gibbs Free Energy signs
There are two other cases where temperature has to be considered. If enthalpy is positive but entropy is also positive, at high temperatures the reaction would have a negative change in free energy and is spontaneous; if enthalpy is negative but entropy is also negative, at low temperatures the reac...
- Fri Feb 15, 2019 1:04 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Gibbs Free Energy graph
- Replies: 2
- Views: 263
Re: Gibbs Free Energy graph
For the graph, the point of lowest energy is where the reaction reaches equilibrium. Any other energy value will spontaneously move towards that lowest energy state and once equilibrium has been reached energy has to be inputted for it proceed. So your statement is correct, I believe.
- Tue Feb 12, 2019 4:33 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Midterm Homework Questions
- Replies: 2
- Views: 396
Re: Midterm Homework Questions
Everything in the syllabus prior to 4J, or entropy, will be covered. I believe that Gibbs free energy will not be be mentioned on the midterm.
- Tue Feb 12, 2019 10:21 am
- Forum: Thermodynamic Definitions (isochoric/isometric, isothermal, isobaric)
- Topic: Extensive vs Intensive
- Replies: 5
- Views: 571
Re: Extensive vs Intensive
Some examples of intensive and extensive properties include density, which is a value independent of how much material you have, and mass, which is scales with the more substance you have, respectively.
- Tue Feb 12, 2019 10:16 am
- Forum: Third Law of Thermodynamics (For a Unique Ground State (W=1): S -> 0 as T -> 0) and Calculations Using Boltzmann Equation for Entropy
- Topic: Irreversible and Reversible Expansion
- Replies: 2
- Views: 266
Re: Irreversible and Reversible Expansion
Reversible can be thought of as if the system and surroundings are at equilibrium, where any change in volume can be seen as a infinitesimally close to the original thermodynamic state; irreversible tends towards one direction and no small change can restore initial conditions.
- Tue Feb 12, 2019 10:12 am
- Forum: Calculating Standard Reaction Entropies (e.g. , Using Standard Molar Entropies)
- Topic: Assume Ideal Behavior
- Replies: 3
- Views: 1040
Re: Assume Ideal Behavior
Ideal conditions usually implies that you are working with a monoatomic gas particles that elastically collide and thus can be modeled with the ideal gas law.
- Mon Feb 11, 2019 4:38 pm
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Buffers
- Replies: 4
- Views: 810
Re: Buffers
Buffers are typically a solution containing a weak acid and its conjugate base or a weak base and its conjugate acid. Their overall effect, when subjugated to the introduction of either a strong acid or strong acid, is to lessen the degree by which the pH of the solution is affected. For instance, i...
- Mon Feb 11, 2019 4:06 pm
- Forum: Thermodynamic Systems (Open, Closed, Isolated)
- Topic: difference in states
- Replies: 6
- Views: 773
Re: difference in states
For the most part, we work with closed systems since isolated systems are not able to transfer energy or heat and open systems have non-constant variables of pressure, heat, etc.
- Tue Feb 05, 2019 11:42 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Enthalpy of Vaporization
- Replies: 2
- Views: 471
Re: Enthalpy of Vaporization
Essentially, if given the heat required to do so and the moles of substance analyzed. However, this may be different if other values are given.
- Tue Feb 05, 2019 11:37 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: 7th edition 4C.7
- Replies: 2
- Views: 322
Re: 7th edition 4C.7
The units of enthalpy are typically (energy)/(moles), usually either Joules/mole or kiloJoules/mole.
- Tue Feb 05, 2019 11:34 pm
- Forum: Calculating Work of Expansion
- Topic: Focus 4.B Question 5 - Unit Conversion with Ideal Gas Constants
- Replies: 1
- Views: 243
Re: Focus 4.B Question 5 - Unit Conversion with Ideal Gas Constants
L*atm is equivalent to volume times pressure, giving us the fundamental SI units meters 3 * Newtons * meters -2 , equivalent to a newton*meter, or a Joule (W = -P \Delta V). In the case of thermodynamic systems, the questions would have units that would indicate towards what the final answer should ...
- Mon Feb 04, 2019 3:59 pm
- Forum: Thermodynamic Systems (Open, Closed, Isolated)
- Topic: Closed and Isolated
- Replies: 10
- Views: 747
Re: Closed and Isolated
Cells in this case would be seen exchanging molecules of CO2 and O2 while also having freely exchanging energy, making it an open system.
- Mon Feb 04, 2019 3:57 pm
- Forum: Entropy Changes Due to Changes in Volume and Temperature
- Topic: residual entropy
- Replies: 3
- Views: 347
Re: residual entropy
Residual entropy is mostly considered in situations where all types of entropy is removed, such as the case of cooling molecules to almost 0 K. Under these conditions, the particles are considered motionless and thus we ignore aspects like entropy associated with gaseous phase particles, etc.
- Mon Feb 04, 2019 3:53 pm
- Forum: Thermodynamic Definitions (isochoric/isometric, isothermal, isobaric)
- Topic: Degeneracy
- Replies: 8
- Views: 746
Re: Degeneracy
A good equation mentioned today in lecture for calculating the number of possible degenerate state was the formula 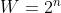 , useful to determining all possible microstates of
, useful to determining all possible microstates of 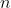 different and separate particles.
different and separate particles.
- Mon Feb 04, 2019 3:50 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: ^U=q+w
- Replies: 1
- Views: 191
Re: ^U=q+w
These equations are theoretically derived as shown during lecture, typically involving work (which is equivalent to the negative integral of volume times pressure) and enthalpy (related the internal energy of the system) with assumptions of an idealized reversible thermodynamic system.
- Thu Jan 31, 2019 10:40 am
- Forum: Thermodynamic Systems (Open, Closed, Isolated)
- Topic: Surroundings
- Replies: 11
- Views: 949
Re: Surroundings
Typically, the distinction between the object of interest and surroundings is the reaction vessel where the pertinent reaction is occurring and the rest of the universe. There are also cases where there are arbitrary boundaries made to distinguish the system from the rest of the universe, used more ...
- Thu Jan 31, 2019 10:36 am
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: 4A.13, 7th ed
- Replies: 1
- Views: 172
Re: 4A.13, 7th ed
The units of Celsius and Kelvin should be interchangeable in this case, they are the same unit just shifted relative to each other.
- Tue Jan 29, 2019 3:37 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Isolated systems
- Replies: 10
- Views: 814
Re: Isolated systems
If needed, say there is a gaseous phase reactant or product in the system, the question would typically make a note to say either pressure or temperature is constant.
- Thu Jan 24, 2019 8:35 pm
- Forum: Phase Changes & Related Calculations
- Topic: Why steam at 100 C burns more than liquid at 100 C
- Replies: 2
- Views: 583
Re: Why steam at 100 C burns more than liquid at 100 C
Yes, steam at 100 degrees Celsius first undergoes a phase change from gas to liquid while remaining at the same temperature, releasing heat via condensation. Now, you are left with water at 100 Celsius which releases energy as same as the sample that started off as water at 100 degrees Celsius. This...
- Thu Jan 24, 2019 8:32 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: 7th Edition 4A.7
- Replies: 1
- Views: 205
Re: 7th Edition 4A.7
In this question, the entire system is consisting of both the copper pot and water inside the copper. You must treat heat flow going to the copper pot, as it also changes in temperature as does the water inside.
- Mon Jan 21, 2019 11:54 am
- Forum: Bronsted Acids & Bases
- Topic: protonation and deprotonation
- Replies: 2
- Views: 612
Re: protonation and deprotonation
Protonation is the process of losing a proton, making a solution more acidic; deprotonation is the converse process. The process of determining protonation would be [conjugate base concentration]/[initial acid concentration]*100%, while deprotonation is [conjugate acid concentration]/[initial base c...
- Tue Jan 15, 2019 11:53 pm
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Negative pH
- Replies: 12
- Views: 1577
Re: Negative pH
For most intents, I think we will not be using such cases of superacids, as these are rather rare anomalies as compared to the scope of Chem 14B.
- Tue Jan 15, 2019 11:51 pm
- Forum: General Science Questions
- Topic: SIG FIGS
- Replies: 5
- Views: 941
Re: SIG FIGS
The reason for this sig fig difference in calculations related to either pH or pOH can be seen on the Chem 14B website, under the link labeled "Everything you want to know about Significant Figures". Here, the concept of how floating point number is made is shown with respect to the mantis...
- Mon Jan 14, 2019 8:56 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Temperature and the equilibrium constant
- Replies: 2
- Views: 302
Re: Temperature and the equilibrium constant
As mentioned before, temperature is the only parameter that affects the equilibrium constant; this occurs as a result of how temperature actually affects the internal energy of the system, shifting the potential and kinetic energies of involved reactants and products.
- Mon Jan 14, 2019 8:54 pm
- Forum: Non-Equilibrium Conditions & The Reaction Quotient
- Topic: Maintaining relation between [H3O+] and [OH-]
- Replies: 2
- Views: 492
Re: Maintaining relation between [H3O+] and [OH-]
The specific process by which this happens was discussed in class today by Dr. Lavelle, autoprotolysis. Water (H2O) occasionally, and spontaneously, pronates other water molecules, thus forming hydronium (H3O+) and hydroxide (OH-) ions.
- Fri Jan 11, 2019 10:09 am
- Forum: Non-Equilibrium Conditions & The Reaction Quotient
- Topic: defining Q
- Replies: 6
- Views: 594
Re: defining Q
Yes, this implies that the reaction will need some defined period of time to reach dynamic equilibrium.
- Mon Jan 07, 2019 5:49 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Equilibrium Concentration vs Pressure
- Replies: 6
- Views: 444
Re: Equilibrium Concentration vs Pressure
If a reaction is given with reactants and products solely in the gas phase, we would use Kp and use partial pressures derived from concentrations and the ideal gas law. Otherwise, Kc would be used with the concentrations involved.
- Fri Nov 30, 2018 2:21 pm
- Forum: Lewis Acids & Bases
- Topic: Strength
- Replies: 3
- Views: 358
Re: Strength
The difference between strong and weak acids/bases is dependent on their dissociation of either hydrogen or hydroxide ions, respectively. Strong acids typically have anions with a high degree of electronegativity, allowing for their hydrogen ions to be loosely bound and prone to disassociate. Strong...
Re: Naming
Aqua- is used where there is water molecules inside the coordination sphere. For instance, [Cr(NH3)3(H2O)3]Cl3 is triamminetriaquachromium(III) chloride.
- Thu Nov 29, 2018 11:05 pm
- Forum: Biological Examples
- Topic: Myoglobin vs Hemoglobin
- Replies: 2
- Views: 197
Re: Myoglobin vs Hemoglobin
Hemoglobin is made from four myoglobin-like subunits that come together via their quaternary structure. The way these four proteins come together have no bearing on the heme group, which binds with the oxygen.
- Thu Nov 29, 2018 7:45 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: bond angle
- Replies: 6
- Views: 524
Re: bond angle
VSEPR does not have methods to find the exact bond angle magnitude (this is determined experimentally), it can tell you the shape and range of what the bond angle could be. Therefore, for the test, you would be only able to write down <120 degrees.
- Thu Nov 29, 2018 7:43 pm
- Forum: Bronsted Acids & Bases
- Topic: HCl Acid
- Replies: 4
- Views: 457
Re: HCl Acid
If HCl is not specified as aqueous, it is in its solid, powdered form, where it is not considered an acid.
- Thu Nov 29, 2018 7:42 pm
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: Chelating ligands
- Replies: 1
- Views: 77
Re: Chelating ligands
Typically chelating ligands have multiple sites that allow for cations to bind, therefore allowing for repetition and stronger binding.
- Thu Nov 29, 2018 12:09 am
- Forum: Interionic and Intermolecular Forces (Ion-Ion, Ion-Dipole, Dipole-Dipole, Dipole-Induced Dipole, Dispersion/Induced Dipole-Induced Dipole/London Forces, Hydrogen Bonding)
- Topic: Melting Points
- Replies: 2
- Views: 297
Re: Melting Points
The first molecule possesses iodine atoms, which are substantially larger than the fluorine atoms found in the second molecule. With a larger atomic number, iodine has more electrons and a larger electron cloud. Iodine, therefore, has a larger polarizability value than fluorine and forces a group of...
- Thu Nov 29, 2018 12:04 am
- Forum: Polarisability of Anions, The Polarizing Power of Cations
- Topic: polarity of ClF3
- Replies: 1
- Views: 2525
Re: polarity of ClF3
To fully understand the polarity of the CIF 3 molecule, we would have to realize the asymmetric distribution of atoms around the central carbon, as well as the differences in dipole moment noted between the C-I bond and the C-F bonds. Combining these two aspects, there is a net dipole in the directi...
- Thu Nov 29, 2018 12:00 am
- Forum: Determining Molecular Shape (VSEPR)
- Topic: VSEPR model
- Replies: 1
- Views: 236
Re: VSEPR model
VSEPR integrates the lone pairs, and different bond types to give more information than a Lewis structure would about the 3D configuration of a molecule.
- Wed Nov 28, 2018 11:58 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Polarity of CH2Cl2
- Replies: 2
- Views: 618
Re: Polarity of CH2Cl2
This Lewis structure does not reflect the 3D structure of CH 2 Cl 2 , which is tetrahedral. With this arrangement, no matter what, at least two corners of the tetrahedron has chlorine atoms. This, along with the difference in electronegativity of chlorine and carbon, produces a net nonzero dipole mo...
- Wed Nov 28, 2018 10:21 pm
- Forum: Hybridization
- Topic: Delocalized Pi Bonding
- Replies: 3
- Views: 304
Re: Delocalized Pi Bonding
Delocalized pi bonding is essentially the sharing of a pi bond over more than two nuclei. This can be prominently be seen with benzene, which shares 3 pi bonds over 6 carbon nuclei and contributes to the ring of electron cloud density characteristic of benzene.
- Wed Nov 28, 2018 10:19 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: How to identify polarity
- Replies: 2
- Views: 379
Re: How to identify polarity
Polar molecules have one thing in common: a net nonzero dipole moment. To determine the dipole moment, one must consider the spatial distribution of the atoms and electron clouds, as well as the differences in electronegativity between the bonded atoms. For instance, in the case of H 2 O, both the e...
- Wed Nov 28, 2018 9:31 pm
- Forum: Dipole Moments
- Topic: Magnitude
- Replies: 2
- Views: 259
Re: Magnitude
Magnitude is meant as the strength of the dipole, usually given in units of debyes.
- Wed Nov 28, 2018 8:31 pm
- Forum: Lewis Structures
- Topic: Lewis Structure Use
- Replies: 1
- Views: 227
Re: Lewis Structure Use
Yes, but for ionic lewis structures, there are brackets around both the anion and cation with charge displayed to indicate that it is an ionic compound.
- Wed Nov 28, 2018 2:04 pm
- Forum: Dipole Moments
- Topic: Lone pairs when determining hybridization
- Replies: 3
- Views: 334
Re: Lone pairs when determining hybridization
Lone pairs are considered separate from one another, this is apparent with the sp3 hybridization of H2O, which has two lone pairs and two bonds.
- Wed Nov 28, 2018 1:17 pm
- Forum: Interionic and Intermolecular Forces (Ion-Ion, Ion-Dipole, Dipole-Dipole, Dipole-Induced Dipole, Dispersion/Induced Dipole-Induced Dipole/London Forces, Hydrogen Bonding)
- Topic: 3F.5 part b (7th Ed.) Melting point
- Replies: 5
- Views: 761
Re: 3F.5 part b (7th Ed.) Melting point
Butanol is a somewhat polar molecule, as evident by the presence of its alcohol functional group (-OH), whereas diethyl ether is a non-polar molecule. The stronger intermolecular forces that come along with butanol as a result of its polar nature make it have a higher melting point than diethyl ether.
- Wed Nov 28, 2018 11:12 am
- Forum: Hybridization
- Topic: hybridized orbitals
- Replies: 2
- Views: 338
Re: hybridized orbitals
In most cases, hybridized orbitals occur as the optimal, lowest energy state configuration a molecule can have; however, some examples exist of molecules whose un-hybridized orbitals are at a lower energy state than having potentially hybridized orbitals (H2S).
- Wed Nov 28, 2018 12:32 am
- Forum: Formal Charge and Oxidation Numbers
- Topic: Formal Charge vs Partial charge
- Replies: 4
- Views: 2296
Re: Formal Charge vs Partial charge
Formal charge is determined by the formula FC = [# of valence electrons on atom] – [non-bonded electrons + number of bonds]; this is useful in determining whether or not a molecule is stable. Partial charge is a notation used to indicate that a molecule has an an asymmetrical distribution of charge ...
- Wed Nov 28, 2018 12:27 am
- Forum: Interionic and Intermolecular Forces (Ion-Ion, Ion-Dipole, Dipole-Dipole, Dipole-Induced Dipole, Dispersion/Induced Dipole-Induced Dipole/London Forces, Hydrogen Bonding)
- Topic: ionic molecule
- Replies: 3
- Views: 350
Re: ionic molecule
Ionic compounds still have London dispersion forces occurring, since the presence of a polarizable electron cloud allows for it. However, the primary intermolecular force present will be that ionic attraction.
- Sat Nov 17, 2018 7:40 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Polarity
- Replies: 3
- Views: 392
Re: Polarity
Polar compounds typically have significant dipole moments and dissolve in the most ubiquitous polar solvent, water. They tend to also have higher melting and boiling points than nonpolar compounds with molecular weights similar to them. Nonpolar compounds lack dipole moments and thus don't dissolve ...
- Fri Nov 16, 2018 3:06 pm
- Forum: Interionic and Intermolecular Forces (Ion-Ion, Ion-Dipole, Dipole-Dipole, Dipole-Induced Dipole, Dispersion/Induced Dipole-Induced Dipole/London Forces, Hydrogen Bonding)
- Topic: H-Bonding
- Replies: 8
- Views: 2263
Re: H-Bonding
Hydrogen bonding is the result of an especially polar bond occurring between the highly electronegative atoms of the upper right section of the periodic table (nitrogen, oxygen, and fluorine) and hydrogen. For most compounds that contain hydrogen, for instance hydrocarbons, hydrogen bonding is not p...
- Fri Nov 16, 2018 3:01 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: bonds
- Replies: 2
- Views: 302
Re: bonds
Sigma bonds are the first type of bond to form (single bonds) in chemical reactions, of which orbitals bond end-to-end and allow for rotation along the internuclear axis. Pi bonds are the second type of bond to form (double and triple bonds) in chemical reactions, of which orbitals bond above or bel...
- Thu Nov 15, 2018 2:54 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: VSEPR forms
- Replies: 3
- Views: 284
Re: VSEPR forms
VESPR shapes are formed more specifically to maximize repulsion between the regions of electron cloud density.
- Fri Nov 09, 2018 2:04 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: VSEPR of H2O
- Replies: 1
- Views: 197
Re: VSEPR of H2O
H2O is not linear because in addition to its 2 bonding pair electrons, it also has 2 lone pair electrons that distort the shape of the atom to resemble an exaggerated tetrahedron. This explains the bent shape of H2O and the presence of a 104.5 degree H-O-H bond angle.
- Fri Nov 09, 2018 1:13 pm
- Forum: Dipole Moments
- Topic: Induced Dipole Meaning
- Replies: 2
- Views: 381
Re: Induced Dipole Meaning
Dipole is defined as the following: "a pair of equal and oppositely charged or magnetized poles separated by a distance." Induced dipole occurs as a result of the polarizability of an electron cloud of a molecule. When an electrically neutral molecule is in close proximity of an ion, the c...
- Fri Nov 09, 2018 1:03 pm
- Forum: Bond Lengths & Energies
- Topic: boiling points
- Replies: 4
- Views: 454
Re: boiling points
Typically, molecules with stronger intermolecular forces tend to have higher melting and boiling points, with hydrogen bonding strength > dipole-dipole interaction > van der Waals interaction.
- Wed Nov 07, 2018 12:58 pm
- Forum: Dipole Moments
- Topic: Dispersion strengths of larger atoms
- Replies: 5
- Views: 397
Re: Dispersion strengths of larger atoms
The general trend for dispersion forces is indeed related to the number of electrons tied to the atom or molecule. Therefore for larger atoms or molecules, the more electrons they have and the greater amount of dispersion forces present.
- Sat Nov 03, 2018 2:50 pm
- Forum: SI Units, Unit Conversions
- Topic: What is a formula unit?
- Replies: 1
- Views: 995
Re: What is a formula unit?
Basically yes. A formula unit indicates the lowest whole number ratio of ions in an ionic compound, as contrasted to molecules, which are composed of two or more elements covalently bonded.
- Sat Nov 03, 2018 1:18 am
- Forum: Trends in The Periodic Table
- Topic: Ionic Radius
- Replies: 3
- Views: 356
Re: Ionic Radius
The Z-effective of Ca 2+ is greater than that of Na+, which is to say the electrostatic attraction between the remaining electrons of Ca 2+ and protons of the nucleus eclipses the electrostatic interaction measured in Na+.
- Thu Nov 01, 2018 2:10 pm
- Forum: *Black Body Radiation
- Topic: Black Body
- Replies: 4
- Views: 2457
Re: Black Body
Given that blackbody radiation was observed as a macroscopic effect, the generalizations produced by classic blackbody radiation theory holds true for classic mechanics. However, if we were to consider the quantized nature of matter, increasingly specific analyses of blackbody radiation fails at the...
- Wed Oct 31, 2018 3:02 pm
- Forum: Octet Exceptions
- Topic: Radical Compounds
- Replies: 1
- Views: 386
Re: Radical Compounds
Radical compounds form in the context of redox reactions, in which a certain chemical species is given an unpaired electron. A prominent example (and one mentioned in class) is the methyl radical, which forms from the incomplete combustion of ethane.
- Tue Oct 30, 2018 10:51 pm
- Forum: Lewis Structures
- Topic: Stable Lewis Structures
- Replies: 4
- Views: 395
Re: Stable Lewis Structures
The Lewis structure version that has the more optimized combination of atoms with formal changes equal to 0 or as closest to 0 would be the more stable Lewis structure.
- Tue Oct 30, 2018 10:47 pm
- Forum: Lewis Structures
- Topic: Valence Electrons
- Replies: 7
- Views: 677
Re: Valence Electrons
An example of an element being bonded with more than 8 valence electrons shared is phosphorus in the compound phosphorus pentachloride (PCl5), which forms an expanded octet to accommodate this change.
- Mon Oct 29, 2018 11:07 am
- Forum: Properties of Light
- Topic: Speed of Light
- Replies: 1
- Views: 187
Re: Speed of Light
Light slows down when in any medium with some amount of matter (water, gas, etc.) because light particles would interact with atoms, get re-emitted, and take a longer period of time to traverse the same distance it would normally travel in a vacuum. For calculating the speed of light through other m...
- Fri Oct 26, 2018 11:38 am
- Forum: Ionic & Covalent Bonds
- Topic: Ionization Energy vs. Electronegativity
- Replies: 5
- Views: 554
Re: Ionization Energy vs. Electronegativity
Ionization energy is the energy required to remove an electron from any atom to turn it into an ion. Electronegativity is a measure of how strongly an atom attracts electrons.
- Fri Oct 26, 2018 11:36 am
- Forum: Electron Configurations for Multi-Electron Atoms
- Topic: Valence Electrons
- Replies: 3
- Views: 264
Re: Valence Electrons
It is a good rule-of-thumb to assume most atoms of the p-block elements want a full octet, or eight electrons in their valence shell. There are exceptions to this eight electron rule, including hydrogen, which tends have 2 electrons in its valence shell once full.
- Tue Oct 23, 2018 3:01 pm
- Forum: Properties of Light
- Topic: Is light in waves or photons?
- Replies: 10
- Views: 972
Re: Is light in waves or photons?
Photons are the elementary particle that is responsible for carrying the electromagnetic force and manifests in electromagnetic radiation. Photons exhibit wave-particle duality, so light is carried by photons which have wave-like and particle-like tendencies.
- Tue Oct 23, 2018 2:10 pm
- Forum: Quantum Numbers and The H-Atom
- Topic: Quantum Numbers
- Replies: 3
- Views: 354
Re: Quantum Numbers
n: the energy level (goes from n = 0,1,2,...) l: the subshell (goes from l = n-1, n-2,...0) m l : the orbital (goes from m l = l, l-1,...-l+1,-1) m s : one of the two electrons that may be within an orbital (either +1/2 or -1/2) First 3 quantum numbers, when expressed as parameters of the wave funct...
- Mon Oct 22, 2018 3:37 pm
- Forum: Properties of Electrons
- Topic: Difference between electron's particle like and wave like characteristics
- Replies: 5
- Views: 387
Re: Difference between electron's particle like and wave like characteristics
From the classic double slit diffraction experiment carried out using an electron beam, it was proven that individual electrons would make contact at singular points on the other side (demonstrating a particle-like nature) but given sufficient time and electrons passing through, the resulting patter...
- Fri Oct 19, 2018 11:04 am
- Forum: Photoelectric Effect
- Topic: Post-Module Assessment Q12
- Replies: 1
- Views: 259
Re: Post-Module Assessment Q12
The answer is C: incoming, large. It is because the energy that is exciting the electron and causing it to be ejected is coming from the incident (incoming) light from some given light source, with photons of a given energy greater than the work function to actually eject the electrons.
- Thu Oct 18, 2018 2:38 pm
- Forum: Electron Configurations for Multi-Electron Atoms
- Topic: Electron configuration
- Replies: 2
- Views: 285
Re: Electron configuration
Silver is among the elements (other examples include copper and gold) with special electron configurations, where it is more energetically stable to have a full d-shell rather than a full s-shell. This is why silver's electron configuration is [Kr] 4d^10 5s^1 rather than [Kr] 4d^9 5s^2.
- Wed Oct 17, 2018 11:24 am
- Forum: Properties of Light
- Topic: color of light
- Replies: 11
- Views: 1091
Re: color of light
Prof. Lavelle has made measurements of light using the wavelength of light, for instance when he mentioned that the wavelengths of violet and red light are 400 and 700 nm, respectively. Wavelength is typically used to determine the color of light.
- Wed Oct 17, 2018 11:21 am
- Forum: Photoelectric Effect
- Topic: SI and equation units
- Replies: 2
- Views: 311
Re: SI and equation units
When in doubt, you can attempt to use dimensional analysis to double-check that you are using the right units. For instance, if you are given Planck's constant's but you forgot the units of it, you can use de Broglie's equation to back-solve for what units Planck's constant is.
- Wed Oct 17, 2018 11:18 am
- Forum: Lewis Structures
- Topic: Radicals
- Replies: 2
- Views: 275
Re: Radicals
The idea of a free radical is not necessarily restricted around even or odd number of valence electrons it has; rather it is dependent on an atom having a "free" electron that is highly unstable and this electron seeks to form a bond by taking an electron from another atom/molecule. Diamag...
- Tue Oct 09, 2018 10:25 pm
- Forum: SI Units, Unit Conversions
- Topic: Ways to remember prefixes
- Replies: 7
- Views: 4884
Re: Ways to remember prefixes
Honestly there is no easy way to go about this, the best way is to brute force memorization of the set of prefixes. Maybe use flashcards?
- Tue Oct 09, 2018 10:19 pm
- Forum: Limiting Reactant Calculations
- Topic: Percent Yield [ENDORSED]
- Replies: 13
- Views: 2899
Re: Percent Yield [ENDORSED]
Theoretical is the value that you obtain from doing stoichiometric calculations and obtaining a number for the maximum possible amount of product. Actual is the value you obtain experimentally, as a result of doing the experiment in a real world setting. In such a situation, the reaction being analy...
- Tue Oct 09, 2018 10:14 pm
- Forum: Properties of Light
- Topic: Microwaves
- Replies: 1
- Views: 150
Re: Microwaves
The way that microwaves work to heat up food is that it interacts in a special way with water. The resonance frequency of water is within the microwave range, so when microwaves are applied to a sample containing water, the water molecules begin to vibrate and produce heat on the macroscopic scale. ...
- Thu Oct 04, 2018 9:52 pm
- Forum: Molarity, Solutions, Dilutions
- Topic: Question Self-Test E.3B
- Replies: 1
- Views: 187
Re: Question Self-Test E.3B
The note is making the statement that there is no need to start from averaging the atomic weights of the two predominant isotopes of copper, then multiplying by Avogadro's constant to get molar mass. Instead it is suggesting that you can just take the molar masses of copper-63 and copper-65 and take...
- Thu Oct 04, 2018 9:47 pm
- Forum: Accuracy, Precision, Mole, Other Definitions
- Topic: F 5 Percent Composition
- Replies: 4
- Views: 450
Re: F 5 Percent Composition
Yea, for this question it asks for the mass percentage composition which is basically asking for the mass percentages of all elements present in l-carnitine. It can be easy to miss that one word that gives you the entire question, I did that in my first reading of the question too.
- Wed Oct 03, 2018 5:08 pm
- Forum: Balancing Chemical Reactions
- Topic: Stoichiometric coefficients
- Replies: 3
- Views: 206
Re: Stoichiometric coefficients
For doing stoichiometric calculations, typically reactants/products with a coefficient of one are not written with the number 1, as it is implied by convention there is one mole of that reactant/product. I personally have not seen the number 1 written down by any previous chemistry teacher.

