Search found 30 matches
- Mon Dec 10, 2018 12:57 am
- Forum: Interionic and Intermolecular Forces (Ion-Ion, Ion-Dipole, Dipole-Dipole, Dipole-Induced Dipole, Dispersion/Induced Dipole-Induced Dipole/London Forces, Hydrogen Bonding)
- Topic: Boiling Point
- Replies: 4
- Views: 1213
Re: Boiling Point
You should look at the bond length. Longer bond length means weaker bonds which can be broken with lower energy (lower boiling point). Smaller bond lengths are stronger and thus require more energy (higher boiling point).
- Mon Dec 10, 2018 12:54 am
- Forum: Properties of Light
- Topic: Snail velocity final problem
- Replies: 3
- Views: 5453
Re: Snail velocity final problem
Do you remember the exact wording of the question? What info was given about the gamma ray? Would it have been possible to find E(photon) using E=hv and then using that as the Ek for the snail?
- Mon Dec 10, 2018 12:41 am
- Forum: Properties & Structures of Inorganic & Organic Acids
- Topic: Why HClO2 is a stronger acid than HBrO2?
- Replies: 6
- Views: 12901
Re: Why HClO2 is a stronger acid than HBrO2?
Does this apply to HNO3 and HNO2 as well? I said HNO3 is stronger because the extra oygen in the molecule helps stabilize the charge from the lone pair.
- Mon Dec 10, 2018 12:35 am
- Forum: Properties & Structures of Inorganic & Organic Acids
- Topic: Strengths of H2S vs H2Se
- Replies: 4
- Views: 9179
Re: Strengths of H2S vs H2Se
LDF is found in every bond because its the most basic form of molecular attraction. Since electrons are constantly moving, at any given time, one atom will be more electronegative than the other atom. Thus, there will always be some degree of induced dipole-induced dipole (also known as LDF).
- Mon Dec 10, 2018 12:18 am
- Forum: Administrative Questions and Class Announcements
- Topic: Discussion Posts
- Replies: 2
- Views: 583
Discussion Posts
How many of these discussion posts do we need to have? And does this week count towards them?
- Mon Dec 10, 2018 12:16 am
- Forum: Biological Examples
- Topic: Cisplatin
- Replies: 4
- Views: 769
Re: Cisplatin
Its a Platinum atom bonded to two Cl atoms and 2 NH3 atoms. The cis also implies that the Cl are on the same side. At least that's what my TA said.
- Sun Nov 25, 2018 10:31 pm
- Forum: Hybridization
- Topic: Test 3
- Replies: 7
- Views: 897
Re: Test 3
Do you know what topics will be covered? I know VSEPR, hybridization, bond angles & shapes will be on the test but am I missing anything else?
- Sun Nov 25, 2018 10:23 pm
- Forum: Properties of Light
- Topic: Speed of light
- Replies: 13
- Views: 3898
Re: Speed of light
I've been using 3.0X10^8 m/s but just to be safe, use the constants he provides on the formula sheet.
- Sun Nov 25, 2018 10:18 pm
- Forum: Octet Exceptions
- Topic: Radicals
- Replies: 2
- Views: 596
Re: Radicals
From what I remember, my TA said it shouldn't matter for this class. Just place the unpaired electron in a way that the formal charge is as close to 0 as possible.
- Sun Nov 18, 2018 9:34 pm
- Forum: Sigma & Pi Bonds
- Topic: Visualizing sigma and pi bonds
- Replies: 7
- Views: 793
Re: Visualizing sigma and pi bonds
I liked this Khan Academy video: https://www.khanacademy.org/science/organic-chemistry/gen-chem-review/hybrid-orbitals-jay/v/pi-bonds-and-sp2-hybridized-orbitals
This other video was also helpful and showed the bonds in 3D: https://www.youtube.com/watch?v=voIpywwj2Ks
This other video was also helpful and showed the bonds in 3D: https://www.youtube.com/watch?v=voIpywwj2Ks
- Sun Nov 18, 2018 9:28 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Types of Bonds
- Replies: 4
- Views: 475
Re: Types of Bonds
Sigma bonds are the initial and strongest bonds formed between two atoms, whereas pi bonds are the subsequent bonds (2nd and 3rd) formed between the same atoms. For example, in O2 (double bond), the first bond created would be a sigma bond and the second one would be a pi bond. In N2 (triple bond), ...
- Sun Nov 18, 2018 8:58 pm
- Forum: Hybridization
- Topic: Sigma/Pi bonds
- Replies: 6
- Views: 866
Re: Sigma/Pi bonds
Identifying the bonds allows you to determine bond strengths and lenghts (not the exact number but relative to other molecules/bonds).
- Sun Nov 11, 2018 10:49 pm
- Forum: Electronegativity
- Topic: Greater Ionic character
- Replies: 3
- Views: 547
Re: Greater Ionic character
Since sulfur is a period below oxygen, it is less electronegative than oxygen. Therefore, the difference in electronegativity between carbon and oxygen will be greater than that of carbon and sulfur.
- Sun Nov 11, 2018 5:42 pm
- Forum: Sigma & Pi Bonds
- Topic: Sigma vs. Pi Bonds
- Replies: 3
- Views: 403
Re: Sigma vs. Pi Bonds
Sigma bonds are the initial and strongest bonds formed between two atoms, whereas pi bonds are the subsequent bonds formed between the same atoms. For example, in O2 (double bond), the first bond created would be a sigma bond and the second one would be a pi bond. In N2 (triple bond), the first woul...
- Sun Nov 11, 2018 5:32 pm
- Forum: Bond Lengths & Energies
- Topic: 3.119 6th ed
- Replies: 2
- Views: 308
Re: 3.119 6th ed
Draw the lewis structure for both molecules and look at what kind of bonds are created (single, double, triple). Based on this you can figure out which bonds are longer and weaker or shorter and stronger; remember, triple bonds are the shortest and strongest because they involve the sharing of more ...
- Sun Nov 04, 2018 11:57 pm
- Forum: Ionic & Covalent Bonds
- Topic: Ionization Energies
- Replies: 13
- Views: 3687
Re: Ionization Energies
How would you determine these trends for diagonal (non-adjacent) elements? For example, between Carbon and Sulfur.
- Sun Nov 04, 2018 11:55 pm
- Forum: Quantum Numbers and The H-Atom
- Topic: Ms quantum number relevancy on the midterm
- Replies: 4
- Views: 473
Re: Ms quantum number relevancy on the midterm
The Ms also helps with understanding Hund's Rule which states: every orbital in a subshell is singly occupied with one electron before any one orbital is doubly occupied. This question popped up on the practice midterm too.
- Sun Nov 04, 2018 11:48 pm
- Forum: Quantum Numbers and The H-Atom
- Topic: Test Quantum Numbers
- Replies: 4
- Views: 552
Re: Test Quantum Numbers
Since n = 5 and l = 1 this would be the 5p orbital. And the p orbitals can hold 6 electrons. Therefore, 6 electrons can have those quantum numbers.
- Mon Oct 29, 2018 12:00 am
- Forum: Wave Functions and s-, p-, d-, f- Orbitals
- Topic: Electron Transitions
- Replies: 1
- Views: 285
Re: Electron Transitions
Did you use this equation:  ? If you use this, which is derived from
? If you use this, which is derived from
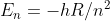 you should get the right answer.
you should get the right answer.
- Sun Oct 28, 2018 11:13 pm
- Forum: Significant Figures
- Topic: Sig Figs on MT
- Replies: 2
- Views: 2511
Re: Sig Figs on MT
Yes, that's correct. My TA mentioned that if we had the right answer but wrong sig figs, we would lose partial credit.
- Sun Oct 28, 2018 11:12 pm
- Forum: Wave Functions and s-, p-, d-, f- Orbitals
- Topic: Heisenberg
- Replies: 2
- Views: 437
Re: Heisenberg
Yes, that is correct! The uncertainty in velocity is the aggregate unknown velocity. For instance, if the velocity is stated to be 1 m/s +/- 0.55 m/s then the velocity could be between 0.45 m/s and 1.55 m/s. Thus, the uncertainty would be 1.55 - 0.45 = 1.10 m/s (or 2 X 0.55).
- Sun Oct 21, 2018 11:33 pm
- Forum: Properties of Light
- Topic: Quantum Equations
- Replies: 3
- Views: 421
Re: Quantum Equations
Based on the question, you can figure out what it's trying to ask and only use the necessary equation. A lot of questions are phrased similarly so doing enough will help you figure out which situations require which equations.
- Sun Oct 21, 2018 11:09 pm
- Forum: Electron Configurations for Multi-Electron Atoms
- Topic: HW problem 1E.1
- Replies: 1
- Views: 247
Re: HW problem 1E.1
The answers shouldn't be different for a Hydrogen atom - (a)-(d) should still increase. It might just be a trick question to see if you understand the concept.
- Sun Oct 21, 2018 11:05 pm
- Forum: Properties of Light
- Topic: Visible Light
- Replies: 5
- Views: 481
Re: Visible Light
My TA mentioned that it's safe to assume visibe light is between 400-700nm.
- Sun Oct 14, 2018 11:44 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Discussion Points
- Replies: 1
- Views: 261
Re: Discussion Points
You need to post 3 times per week (1pt per post) with either questions or repsonses to questions by Sunday 11:59 PM.
- Sun Oct 14, 2018 11:29 pm
- Forum: Properties of Electrons
- Topic: Wavelength Calculations
- Replies: 5
- Views: 644
Re: Wavelength Calculations
The both are constants. The first number, h, is Planck's constant whereas the second number, c, is the speed of light. And for reference, the formula is 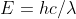
- Sun Oct 14, 2018 11:22 pm
- Forum: Properties of Light
- Topic: 1A.3 7th Edition Question
- Replies: 4
- Views: 460
Re: 1A.3 7th Edition Question
Exactly. Frequency and wavelength have an inverse relationship so as one increases, the other decreases. In this case, decreasing frequency means increasing wavelength. If you were to visualize this, it would mean the waves are getting elongated (less steep). Therefore, the slope decreases as well.
- Sun Oct 07, 2018 10:01 pm
- Forum: Limiting Reactant Calculations
- Topic: Section L, #35
- Replies: 2
- Views: 189
Re: Section L, #35
I was stuck on this one too but figured out that you can add the three reactions. The result will give you: Fe + 2Br2 + Na2CO3 ---> NaBr + CO2 + Fe3O4 Using this equation, you would need to: 1. Balance the equation 2. Calculate the moles of NaBr given you have 2.5t 3. Using the moles of NaBr and the...
- Sun Oct 07, 2018 9:42 pm
- Forum: Limiting Reactant Calculations
- Topic: Determine Limiting Reagent
- Replies: 4
- Views: 3853
Re: Determine Limiting Reagent
If I'm understanding your question correctly, I think the way the professor did it (and in my opinion the more intuitive way) is to calculate the moles of the reactants and then compare them using the equation. This method is pretty quick and sets you up to answer other questions the problem may pos...
- Sun Oct 07, 2018 9:11 pm
- Forum: Limiting Reactant Calculations
- Topic: Question M11
- Replies: 2
- Views: 366
Re: Question M11
The first step woud be to find the number of moles of O2 and P4 in the reaction given that we have 5.77g of each. For example, since the molar mass of O2 is 32g/mol and we have a sample with 5.77g, there will be 5.77g / (32g/mol) = 0.180 mol of O2. Similarly, you can calculate that there are 0.0465 ...

