For example:
Search found 68 matches
- Wed Mar 13, 2019 12:03 am
- Forum: General Rate Laws
- Topic: reaction rate(s)?
- Replies: 4
- Views: 725
Re: reaction rate(s)?
The rate law is an equation that gives you the reaction rate.
For example:
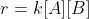
 is the rate, and
is the rate, and 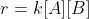 is the rate law.
is the rate law.
For example:
- Wed Mar 13, 2019 12:02 am
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Test 2 Cell Diagram
- Replies: 3
- Views: 492
Re: Test 2 Cell Diagram
a) You use a comma when there are two species in the same phase. For example, if 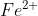 is being oxidized to
is being oxidized to 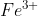 in the anode, you use a comma to separate them in the cell diagram, instead of a vertical line.
in the anode, you use a comma to separate them in the cell diagram, instead of a vertical line.
b} Yes, you use a conductor (usually platinum) when no solid is present.
b} Yes, you use a conductor (usually platinum) when no solid is present.
- Tue Mar 12, 2019 11:56 pm
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: Test 2
- Replies: 5
- Views: 544
Re: Test 2
You have to use the Van't Hoff equation: ln(\frac{k_{2}}{k_{1}})=-\frac{\Delta H}{R}(\frac{1}{T_{2}}-\frac{1}{T_{1}}) Plug in values: ln(\frac{k_{2}}{10^{-14}})=-\frac{58000}{8.314}(\frac{1}{303.15}-\frac{1}{298.15}) Solve for k_{2} : k_{2}=1.3\times 10^{-14} pk_{2}=-...
- Tue Mar 12, 2019 11:34 pm
- Forum: First Order Reactions
- Topic: First Order Reactions
- Replies: 3
- Views: 442
Re: First Order Reactions
I think the order of the reaction has to be determined experimentally. You can't just look at a reaction and tell what order it is. 2A -> B + C looks like a second-order reaction but it doesn't have to be one. What if the reaction took place in two steps and involved the intermediate molecules X and...
- Tue Mar 12, 2019 1:21 am
- Forum: Reaction Mechanisms, Reaction Profiles
- Topic: slow step and fast step
- Replies: 3
- Views: 376
Re: slow step and fast step
It's actually the opposite. The fast step does not affect the overall rate because the overall reaction is only as fast as its slowest step. No matter how fast other reactions run, the overall reaction can't be completed until the slowest step is completed. Therefore, the rate of the overall reactio...
- Sun Mar 10, 2019 5:45 pm
- Forum: Second Order Reactions
- Topic: 7E.3 in 7th edition
- Replies: 3
- Views: 717
Re: 7E.3 in 7th edition
125-175=50
0.4(125)=50
The two ways of writing it are equivalent. You must be making a mistake elsewhere.
0.4(125)=50
The two ways of writing it are equivalent. You must be making a mistake elsewhere.
- Sun Mar 10, 2019 5:29 pm
- Forum: Reaction Mechanisms, Reaction Profiles
- Topic: Rate Determining Step
- Replies: 1
- Views: 280
Re: Rate Determining Step
There is no way to figure out which step is fast or slow since this is determined experimentally. The question will always tell you which step is the slowest.
- Sun Mar 10, 2019 5:26 pm
- Forum: Reaction Mechanisms, Reaction Profiles
- Topic: Reaction Profiles
- Replies: 2
- Views: 379
Re: Reaction Profiles
The activation energy doesn't matter. A reaction is spontaneous when the products have lower energy than the reactants, making  negative.
negative.
- Sun Mar 10, 2019 5:23 pm
- Forum: General Rate Laws
- Topic: Reaction Order
- Replies: 5
- Views: 584
Re: Reaction Order
It depends on the sum of all exponents in the rate law. The rate law can only be calculated experimentally.
- Sun Mar 10, 2019 5:18 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Equation I've never seen before?
- Replies: 3
- Views: 410
Re: Equation I've never seen before?
PJ is the partial pressure of a gas and nJ is the number of moles of that gas. Other than that it's just the ideal gas law PV=nRT.
- Sun Mar 10, 2019 5:15 pm
- Forum: Method of Initial Rates (To Determine n and k)
- Topic: Reaction Constant k
- Replies: 2
- Views: 429
Re: Reaction Constant k
I believe temperature is the only thing that affects k. An increase in temperature increases k by increasing the odds of collision between reactant molecules.
- Mon Mar 04, 2019 12:55 am
- Forum: Work, Gibbs Free Energy, Cell (Redox) Potentials
- Topic: Finding n
- Replies: 12
- Views: 1126
Re: Finding n
For example, if one mole of Fe were being oxidized to Fe2+, n would be 2 because 2 moles of electrons would be transferred. If Fe had a coefficient other than one, you would multiply it by 2.
- Mon Mar 04, 2019 12:51 am
- Forum: Work, Gibbs Free Energy, Cell (Redox) Potentials
- Topic: Delta G rules
- Replies: 8
- Views: 781
Re: Delta G rules
The reaction is spontaneous when delta G is negative, nonspontaneous when delta G is positive, and at equilibrium when delta G is 0
- Mon Mar 04, 2019 12:49 am
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Pt
- Replies: 14
- Views: 1357
Re: Pt
You would only put that in your diagram if the question says a platinum electrode is used. You would generally use a platinum electrode when the actual substances you're working with are gases.
- Sun Mar 03, 2019 11:59 pm
- Forum: Balancing Redox Reactions
- Topic: OH-
- Replies: 5
- Views: 578
Re: OH-
When balancing oxygen in basic solution
- Sun Mar 03, 2019 11:58 pm
- Forum: Zero Order Reactions
- Topic: Order distinction
- Replies: 9
- Views: 1107
Re: Order distinction
By the highest power in the reaction quotient
- Sun Feb 24, 2019 4:55 am
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: oxygen, hydrogen, water
- Replies: 1
- Views: 269
Re: oxygen, hydrogen, water
I know oxygen is being reduced on one side and oxidized on the other (its oxidation number is 0 in 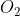 and -2 in
and -2 in 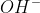 so electrons are going from the left side to the right side). But I'm not really sure why electrons are going one way and not the other. Can someone explain this?
so electrons are going from the left side to the right side). But I'm not really sure why electrons are going one way and not the other. Can someone explain this?
- Sun Feb 24, 2019 4:46 am
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: 4.41 (7th edition)
- Replies: 1
- Views: 212
Re: 4.41 (7th edition)
I think you can look at the individual free energies. The one with a smaller G will be the more stable one. But a negative \Delta G for the reaction tells you the same thing so you could just look at that instead of looking at two numbers. I can't think of any reason why you wouldn't get the same re...
- Sat Feb 23, 2019 4:50 am
- Forum: Balancing Redox Reactions
- Topic: distinguishing between acidic and basic solutions
- Replies: 3
- Views: 433
Re: distinguishing between acidic and basic solutions
All the questions I have seen in the textbook tell you what kind of solution it is
- Sat Feb 23, 2019 4:47 am
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Porous Disk
- Replies: 1
- Views: 316
Re: Porous Disk
1. Because the ions in solution are not exchanging electrons. The solid electrodes are exchanging electrons. And the only way for them to do that is to send electrons through the wire. The ions that are dissolved in solution have already been oxidized/reduced, and do not participate in any more reac...
- Sat Feb 23, 2019 4:34 am
- Forum: Van't Hoff Equation
- Topic: Deriving Equations
- Replies: 3
- Views: 508
Re: Deriving Equations
Can you be more specific about which equations you're talking about?
- Sat Feb 16, 2019 8:03 pm
- Forum: Entropy Changes Due to Changes in Volume and Temperature
- Topic: Adding reaction entropies
- Replies: 8
- Views: 895
Re: Adding reaction entropies
It's also important to remember that \Delta S is not entropy of a system, but the change in entropy as a result of that reaction. So if a reaction happens in multiple steps, and each of those steps causes a change in entropy, adding all those changes together gives you the total change as a result o...
- Sat Feb 16, 2019 7:54 pm
- Forum: Third Law of Thermodynamics (For a Unique Ground State (W=1): S -> 0 as T -> 0) and Calculations Using Boltzmann Equation for Entropy
- Topic: Explaining Boltzmann's Equation
- Replies: 6
- Views: 1396
Re: Explaining Boltzmann's Equation
I found a relatively accessible derivation of the formula here: http://www.eoht.info/page/S+%3D+k+ln+W
- Sat Feb 16, 2019 7:44 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Spontaneous vs boiling point?
- Replies: 4
- Views: 844
Re: Spontaneous vs boiling point?
A reaction is spontaneous when \Delta G<0 \Delta G=\Delta H-T\Delta S \Delta H> 0 for boiling a liquid, because you are putting heat into the reaction. \Delta S> 0 for boiling a liquid, because gases are more disordered than liquids. So to make \Delta G<0 , all you have to do is raise the temperatur...
- Sun Feb 10, 2019 11:59 pm
- Forum: Phase Changes & Related Calculations
- Topic: Heat vs Q
- Replies: 5
- Views: 515
Re: Heat vs Q
the change in energy of the system or delta U is equal to q+W. W is energy transferred to the system by work, while q is the energy transferred by heat.
- Sun Feb 10, 2019 11:55 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Polyprotic acid question 2.0
- Replies: 1
- Views: 195
Re: Polyprotic acid question 2.0
I think the general rule is that you can ignore the second deprotonation if Ka2 is smaller than Ka1/1000.
- Sun Feb 10, 2019 11:52 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: 8.41
- Replies: 1
- Views: 184
Re: 8.41
You would need to use the heat of fusion. heat gained by ice = heat lost by water (heat needed to melt ice)(heat needed to warm melted ice)=(heat lost by water) (heat of fusion for water)(mass of ice)+(mass of melted ice)(heat capacity of water)(x-0)=(mass of water in the glass)(heat capacity of wat...
- Mon Feb 04, 2019 1:42 am
- Forum: Thermodynamic Systems (Open, Closed, Isolated)
- Topic: Thermos
- Replies: 4
- Views: 549
Re: Thermos
A closed system allows energy to leave and enter, but not matter. An isolated system allows neither energy nor matter to leave and enter. In reality, a thermos is a closed system because it does allow for the exchange of small amounts of energy (A hot drink eventually cools down in a thermos). But t...
- Mon Feb 04, 2019 1:33 am
- Forum: Phase Changes & Related Calculations
- Topic: Heat and Condensation
- Replies: 9
- Views: 959
Re: Heat and Condensation
Condensation is the transition from gas to liquid. Gas molecules have lots of energy because they are moving around quickly. To turn them into liquid, you have to slow them down significantly, by removing heat from them. Therefore, condensation releases heat.
- Mon Feb 04, 2019 1:31 am
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: Work
- Replies: 6
- Views: 661
Re: Work
Work is positive (done on the system) if it adds energy to the system. For example, if you increase the pressure of a gas using a piston, you are doing work on the gas because you are adding energy to it. If you heat a gas, you are adding energy to it, therefore you are doing work on it.
- Mon Jan 21, 2019 3:37 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Equilibrium costant
- Replies: 2
- Views: 276
Re: Equilibrium costant
When all your reagents are gases, you can choose whether to use partial pressures or concentrations. But in this case, you have a gas on one side and an aqueous reagent on the other. You obviously can't convert the aqueous concentration to a partial pressure since it's not a gas. And you can't conve...
- Mon Jan 21, 2019 3:33 pm
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Inert Gases
- Replies: 7
- Views: 829
Re: Inert Gases
It's not really the change in pressure that affects the equilibrium, it's the change in concentrations. When you decrease the volume, there is less space for molecules to move around in. It gets more crowded. Let's say you have a reaction that has more moles on the reactants side. If you have a lot ...
- Mon Jan 21, 2019 3:23 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Integrated Exercises 12.109
- Replies: 1
- Views: 192
Re: Integrated Exercises 12.109
I'm not sure if it's the right answer, but my guess is that hydronium and hydroxide ions don't really move through water, they only ionize the water molecules next to them, so the charge moves through the water, not the actual ion. Especially since the question says they "appear to move" f...
- Mon Jan 21, 2019 3:16 pm
- Forum: Non-Equilibrium Conditions & The Reaction Quotient
- Topic: Using Equilibrium Constants to Predict Solubility
- Replies: 2
- Views: 305
Re: Using Equilibrium Constants to Predict Solubility
Exactly. If a reaction has solid reactants and aqueous products, a larger equilibrium constant means the reactants are more soluble.
- Mon Jan 21, 2019 3:15 pm
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Le Chatelier's Principle on Temperature
- Replies: 4
- Views: 507
Re: Le Chatelier's Principle on Temperature
If the reaction is exothermic, heat is released as a result of the reaction. Therefore, lowering heat is like removing a product and the equilibrium shifts toward the products.If the reaction is endothermic, removing heat is like removing a reactant, so the equilibrium shifts toward the reactants.
- Mon Jan 21, 2019 3:13 pm
- Forum: Non-Equilibrium Conditions & The Reaction Quotient
- Topic: Acid Strength
- Replies: 3
- Views: 319
Re: Acid Strength
Ka is the dissociation constant for the acid. The higher the Ka the more completely the acid dissociates. Therefore high Ka corresponds to stronger acid. pKa is the negative log of Ka. So the smaller the pKa the stronger the acid.
- Sun Jan 13, 2019 12:41 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: solids & liquids
- Replies: 5
- Views: 452
Re: solids & liquids
Because solids and liquids don't have concentrations. They're pure substances. So including them makes no sense.
- Sun Jan 13, 2019 12:34 am
- Forum: Ideal Gases
- Topic: Q
- Replies: 5
- Views: 496
Re: Q
Q is the reaction quotient, which is the concentrations of products over the concentrations of reactants, with the coefficients as exponents. K is just the specific value of Q when the reaction is at equilibrium.
- Sun Jan 13, 2019 12:32 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: number 5I.25 7th edition
- Replies: 1
- Views: 177
Re: number 5I.25 7th edition
Since the reaction is not currently at equilibrium you would need to use an ICE table. For the initial concentrations, you would use the mol values provided divided by the volume (5L). Then you use the quadratic formula to find the changes in concentration, which would then give you the final concen...
- Sun Dec 09, 2018 4:30 pm
- Forum: Bohr Frequency Condition, H-Atom , Atomic Spectroscopy
- Topic: determining second n value
- Replies: 1
- Views: 486
Re: determining second n value
Plug in and solve for
- Sun Dec 09, 2018 4:10 pm
- Forum: Limiting Reactant Calculations
- Topic: moles of reagant
- Replies: 4
- Views: 724
Re: moles of reagant
You would use the coefficients to find out exactly how much of one reactant you need to completely react with a given amount of the other reactant.
- Sun Dec 09, 2018 4:08 pm
- Forum: Heisenberg Indeterminacy (Uncertainty) Equation
- Topic: determining uncertainty in momentum
- Replies: 1
- Views: 459
Re: determining uncertainty in momentum
Uncertainty in momentum times uncertainty in position is greater than or equal to planck's constant over 4 pi. You are given the uncertainty in position. Plug it into the equation and solve the inequality.
- Sun Dec 09, 2018 4:06 pm
- Forum: Acidity & Basicity Constants and The Conjugate Seesaw
- Topic: acid rain formula
- Replies: 4
- Views: 520
Re: acid rain formula
Depends on the pollutant in question. Sulfur dioxide is a common example:
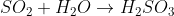
- Sat Dec 01, 2018 7:09 pm
- Forum: Calculating the pH of Salt Solutions
- Topic: The pH of Solutions of Weak Acids and Bases 12.65
- Replies: 5
- Views: 638
Re: The pH of Solutions of Weak Acids and Bases 12.65
a) Compound goes into water. It breaks up into Br - and NH 4 + . Br - doesn't do anything. Some of the NH 4 + release a hydrogen and become NH 3 . Releasing a hydrogen makes the solution acidic. pH falls below 7. b) Breaks up in water. The sodium does nothing. The carbonate is the conjugate base of ...
- Sat Dec 01, 2018 6:53 pm
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: Oxidation Numbers
- Replies: 1
- Views: 231
Re: Oxidation Numbers
You're generally supposed to know the charges on common polyatomic ions. So you should know that CN has a -1 charge. If you don't know the charges beforehand, the name of the compound may also help you figure it out. The (II) in hexacyanoferrate(II) tells you that the iron has a +2 charge. Since the...
- Sat Dec 01, 2018 6:40 pm
- Forum: Calculating pH or pOH for Strong & Weak Acids & Bases
- Topic: Question 12.19 (6th Edition)
- Replies: 1
- Views: 307
Re: Question 12.19 (6th Edition)
Original concentration: 10 x Concentration diluted to 12% of original value: (10 x )(0.12) Original pH: -log(10 x ) pH after dilution: -log((10 x )(0.12)) = -log(10 x ) - log(0.12) Change in pH = (Original pH) - (pH after dilution) = -log(10 x ) - (-log(10 x ) - log(0.12)) Change in pH = -log(10 x )...
- Sun Nov 25, 2018 10:47 pm
- Forum: Hybridization
- Topic: orbital hybridization and polarity
- Replies: 1
- Views: 426
Re: orbital hybridization and polarity
Polarity has to do with asymmetry and difference in electronegativity. If there are polar bonds and the molecule is asymmetrical, then it's polar. Hybridization requires a little bit of thinking. You can think of the central atom having multiple electron "sites". A site can contain a bond ...
- Sun Nov 25, 2018 10:41 pm
- Forum: Dipole Moments
- Topic: Dipole Moments
- Replies: 2
- Views: 308
Re: Dipole Moments
A permanent dipole happens when the molecule contains polar bonds and is asymmetrical. For example, doesn't have a permanent dipole moment because it contains no polar bonds. Carbon tetrachloride doesn't have a permanent dipole moment because it's symmetrical. Chloroform does have a permanent dipole...
- Sun Nov 25, 2018 10:35 pm
- Forum: Lewis Acids & Bases
- Topic: Conjugate Acid and Base
- Replies: 1
- Views: 254
Re: Conjugate Acid and Base
I think you have that backwards. The concept of conjugates exists in Bronsted-Lowry, since it deals with proton exchange. An acid loses a proton and becomes its conjugate base, a base gains a proton becomes its conjugate acid. Lewis acids and bases don't always exchange protons. For example, BF 3 is...
- Sun Nov 18, 2018 10:53 pm
- Forum: Interionic and Intermolecular Forces (Ion-Ion, Ion-Dipole, Dipole-Dipole, Dipole-Induced Dipole, Dispersion/Induced Dipole-Induced Dipole/London Forces, Hydrogen Bonding)
- Topic: Interaction Potential Energy
- Replies: 3
- Views: 423
Re: Interaction Potential Energy
Larger molecules/atoms have larger electron clouds which can be distorted to a greater extent, leading to stronger interactions.
- Sun Nov 18, 2018 10:50 pm
- Forum: Interionic and Intermolecular Forces (Ion-Ion, Ion-Dipole, Dipole-Dipole, Dipole-Induced Dipole, Dispersion/Induced Dipole-Induced Dipole/London Forces, Hydrogen Bonding)
- Topic: Question 6.3 (Sixth Edition)
- Replies: 1
- Views: 877
Re: Question 6.3 (Sixth Edition)
The question is basically asking which compounds experience dipole-dipole interactions. You can eliminate A and E. A is nonpolar because carbon and hydrogen have almost identical electronegativities. E is nonpolar since the chlorines are symmetrically arranged around the carbon. So A and E will not ...
- Sun Nov 18, 2018 10:44 pm
- Forum: Hybridization
- Topic: E- Promotion and Hybridization
- Replies: 2
- Views: 3100
Re: E- Promotion and Hybridization
Electron promotion happens when an electron that used to be in an s orbital is pushed up to a higher energy level to allow for hybridization. For example, carbon has two electrons in 2s and two electrons in 2p. To hybridize, one electron is pushed from 2s up to 2p. Now there is one electron in 2s an...
- Sun Nov 11, 2018 11:55 pm
- Forum: Ionic & Covalent Bonds
- Topic: Ionic character
- Replies: 1
- Views: 151
Re: Ionic character
Bond polarity exists on a spectrum. On one side you have covalent, on the other side is ionic. You could replace covalent with nonpolar and ionic with polar. The more polar a bond gets (more electronegativity difference between the atoms) the closer it gets to the ionic side, so you say it has more ...
- Sun Nov 11, 2018 11:47 pm
- Forum: Interionic and Intermolecular Forces (Ion-Ion, Ion-Dipole, Dipole-Dipole, Dipole-Induced Dipole, Dispersion/Induced Dipole-Induced Dipole/London Forces, Hydrogen Bonding)
- Topic: Bond energy value signs
- Replies: 3
- Views: 499
Re: Bond energy value signs
Because energy is released when a bond forms. Since energy is leaving the system, the sign is negative.
- Sun Nov 11, 2018 11:44 pm
- Forum: Bond Lengths & Energies
- Topic: Bond lengths [ENDORSED]
- Replies: 3
- Views: 499
Re: Bond lengths [ENDORSED]
I think what you're thinking about is atomic radius, which increases as you move down and to the left on the periodic table. As atomic radius increases, the distance between the nuclei of atoms in a bond increases, so the bond gets longer.
- Sun Nov 11, 2018 11:38 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: 7th edition 2E.5
- Replies: 1
- Views: 167
Re: 7th edition 2E.5
First step of drawing a Lewis structure: count your valence electrons. 7 from the chlorine and 6 from each oxygen gives you 19. Since there is a charge of +1, you have 18 valence electrons. Normally, chlorine would only be able to form one bond, since it only has one unpaired electron. But since we ...
- Sun Nov 04, 2018 1:04 pm
- Forum: Electron Configurations for Multi-Electron Atoms
- Topic: 7th edition 2A.11
- Replies: 1
- Views: 284
Re: 7th edition 2A.11
Short answer: transition metals are the spawn of satan and they are out to ruin your life. Long answer: During ionization, 4s electrons are lost before 3d electrons. So if you removed three electrons and ended up with 3d 6 , that means you removed 2 electrons from 4s and 1 electron from 3d. So the o...
- Sun Nov 04, 2018 12:36 pm
- Forum: Lewis Structures
- Topic: Question 3.55 (Sixth Edition)
- Replies: 6
- Views: 630
Re: Question 3.55 (Sixth Edition)
A radical is any molecule/atom that has unpaired valence electrons. Normally you want electrons to be either in bonds or in lone pairs. When an electron is not in a bond or a pair and it's just hanging out by itself, it's really reactive, and you call the molecule/atom a radical. You can draw Lewis ...
- Sun Nov 04, 2018 12:19 pm
- Forum: Electron Configurations for Multi-Electron Atoms
- Topic: Electron configuration when 4s and 3d
- Replies: 3
- Views: 431
Re: Electron configuration when 4s and 3d
You fill the 4s first, just like the Aufbau principle tells you to. The only exceptions are Chromium and Copper. The reason those two are different is that orbitals want to be either full or half-full whenever possible. Aufbau tells you chromium should be [Ar]3d 4 4s 2 , but the actual configuration...
- Sun Oct 28, 2018 9:57 pm
- Forum: Electronegativity
- Topic: Electronegativity vs Electron Affinity
- Replies: 4
- Views: 37073
Re: Electronegativity vs Electron Affinity
They are similar concepts with different applications. Electronegativity is only relevant in bonded atoms. The atom that attracts the electrons more in a bond has higher electronegativity. If the atoms are by themselves the concept of electronegativity is irrelevant. Electron affinity is a property ...
- Sun Oct 28, 2018 9:38 pm
- Forum: Electron Configurations for Multi-Electron Atoms
- Topic: 4s becomes higher energy
- Replies: 3
- Views: 3061
Re: 4s becomes higher energy
The 4s subshell is lower in energy than the 3d subshell, so it fills up first. The 4s becomes higher in energy after filling up, so if you ionize the atom, electrons are lost from the 4s subshell before the 3d subshell. But that's not how I learned it so it just confuses me to think of it that way. ...
- Sun Oct 28, 2018 9:24 pm
- Forum: Trends in The Periodic Table
- Topic: Atomic Radii
- Replies: 2
- Views: 316
Re: Atomic Radii
It actually does, which is why a negative ion is always larger than a neutral atom of the same element. The trend you are talking about is atomic radii getting smaller as you move right in the periodic table. That happens because you're adding protons to the nucleus, which increases the pull on the ...
- Sun Oct 14, 2018 2:31 pm
- Forum: Properties of Electrons
- Topic: Diffraction patterns
- Replies: 9
- Views: 1111
Re: Diffraction patterns
If two waves are in phase (peaks line up with peaks perfectly), the waves will interfere constructively and result in a wave of greater amplitude. If the waves are out of phase by half a wavelength (assuming they have the same wavelength) then they interfere destructively (peaks line up with troughs...
- Sun Oct 14, 2018 2:17 pm
- Forum: Trends in The Periodic Table
- Topic: problem 1.13
- Replies: 3
- Views: 348
Re: problem 1.13
There are essentially two factors that determine ionization energy. There is the attraction between electrons and the nucleus, and the repulsion between electrons and other electrons. As you go from left to right on the periodic table, the number of protons in the nucleus increases, so the attractio...
- Sun Oct 14, 2018 2:04 pm
- Forum: Properties of Light
- Topic: De Broglie Equation
- Replies: 6
- Views: 459
Re: De Broglie Equation
The equation contains the Planck constant, which is usually given in 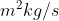 . So you need to plug in masses in kilograms, otherwise the units won't cancel out properly.
. So you need to plug in masses in kilograms, otherwise the units won't cancel out properly.
- Wed Oct 03, 2018 5:34 pm
- Forum: Molarity, Solutions, Dilutions
- Topic: Limiting Reactants
- Replies: 3
- Views: 321
Re: Limiting Reactants
First thing to notice is that it asks for the "net ionic equation." That means you only include reactants and products that are involved in a permanent or irreversible reaction. If you remember solubility rules, you know that any ionic compound containing nitrate or sodium is soluble in wa...
- Wed Oct 03, 2018 5:22 pm
- Forum: Molarity, Solutions, Dilutions
- Topic: E.9 on homework
- Replies: 4
- Views: 296
Re: E.9 on homework
The question tells you that epsom salt consists of magnesium sulfate heptahydrate. The prefix hepta- means 7. If you weren't given the name of the compound or the chemical formula then you would have no way of knowing the coefficient for water.
- Wed Oct 03, 2018 5:18 pm
- Forum: Balancing Chemical Reactions
- Topic: Stoichiometric coefficients
- Replies: 3
- Views: 199
Re: Stoichiometric coefficients
It's not wrong. It's just unnecessary and unconventional. If you were going to publish a scholarly paper or something that contained chemical reactions, then you would obviously make sure not to include coefficients of 1 since it's against convention. But I can't imagine anyone taking points off for...

