Search found 61 matches
- Thu Mar 14, 2019 12:43 am
- Forum: Student Social/Study Group
- Topic: Key Words
- Replies: 2
- Views: 710
Re: Key Words
Try searching "Joyce". She uploads worksheets!
- Thu Mar 14, 2019 12:28 am
- Forum: Arrhenius Equation, Activation Energies, Catalysts
- Topic: 15.61 (6th edition)
- Replies: 1
- Views: 257
Re: 15.61 (6th edition)
They got this by using the equation ln(k) = -Ea/(R*T). Because you are finding the difference in two different k values, you would use ln(k')-ln(k), which is ln(k'/k). On the other side of the equation, you would have Ea (which is constant) and R (which is constant) and T (which changes). This means...
- Thu Mar 14, 2019 12:21 am
- Forum: Arrhenius Equation, Activation Energies, Catalysts
- Topic: 15.67 (6th ed.)
- Replies: 1
- Views: 256
Re: 15.67 (6th ed.)
I used the method of directly plugging in the values of the Ea into the formula of A*e -Ea/(R*T) to get the ratio of e^x for each of the reactions. I divided the second e^x number (Ea=125) by the first e^x number (Ea=75) and got the factor the k increases by, as A remains constant in both re...
- Thu Mar 07, 2019 12:10 pm
- Forum: General Rate Laws
- Topic: 6th edition 15.17 why is c independent of the rate?
- Replies: 1
- Views: 212
Re: 6th edition 15.17 why is c independent of the rate?
If you look at experiment 1 and experiment 4, you see that the concentration of A and B remained the same, while the concentration of C goes from 700 mmol/L to 400 mmol/L. Because concentration of C is the only thing changing, any changes in the initial rate would stem from changes in C, IF the rate...
- Thu Mar 07, 2019 12:05 pm
- Forum: First Order Reactions
- Topic: Using ln[A]
- Replies: 2
- Views: 332
Re: Using ln[A]
You would use ln[A] for first order reactions. For these reactions, rate=k[A]=-1/a * d[A]/dt. If you set a=1 and then you separate the variables, you get k*dt=-d[A]/[A] or (-1/[A])*d[A]. If you integrate both sides, you would get kt=-ln[A], or -kt=ln[A], as the integral of (-1/[A])*d[A] is ln[A]. Th...
- Thu Mar 07, 2019 12:03 am
- Forum: Second Order Reactions
- Topic: deciding which order
- Replies: 2
- Views: 356
Re: deciding which order
If you are given the units of K, you can determine the order of the reaction by looking at those units, as they should cancel out with the reactant's units to get \frac{mol}{L*sec} , which is the unit for the rate of the reaction. You know that the [reactant] is \frac{mol}{L} . For example: Rate con...
- Wed Feb 27, 2019 12:42 am
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: 9.65 6th edition writing chemical equations
- Replies: 1
- Views: 243
Re: 9.65 6th edition writing chemical equations
You can use either the equation for decomposition or composition, as it will yield the same answer. As an example, part (a): If you used the equation of composition, P + 5/2Cl2 -> PCl5, you would find that the entropy of the reaction was negative. This means that at higher temperatures, the reverse ...
- Tue Feb 26, 2019 11:57 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: E^o(cell) vs. Ecell
- Replies: 6
- Views: 13290
Re: E^o(cell) vs. Ecell
Eocell is the standard state cell potential, meaning the condition of [1M] for solutes or 1 bar for gases is met. Ecell is the cell potential at non standard state conditions. You can use the Nernst equation to find Ecell using Eocell.
- Tue Feb 26, 2019 11:49 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Agents and Species
- Replies: 3
- Views: 356
Re: Agents and Species
A reducing agent is the substance that has the ability to reduce other substances, by losing electrons and being oxidized.The reduced species is the thing that is reduced (gain electrons) An oxidizing agent is the substance that has the ability to oxidize other substances, by gaining electrons and b...
- Sun Feb 24, 2019 9:21 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Cell Diagrams
- Replies: 2
- Views: 330
Re: Cell Diagrams
Professor Lavelle used a single horizontal line to represent the interface between phases in contact with each other (including a porous disc/wall) and a double horizontal line to represent the salt bridge.
EDIT: a comma is used when the oxidized and reduced species are in the same phase.
EDIT: a comma is used when the oxidized and reduced species are in the same phase.
- Sun Feb 24, 2019 3:08 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Volts
- Replies: 2
- Views: 273
Re: Volts
Yes, 1V= 1J/Coulomb, so 1 Volt is 1 Joule of Work per Coulomb of charge transferred
- Sun Feb 24, 2019 3:04 pm
- Forum: Work, Gibbs Free Energy, Cell (Redox) Potentials
- Topic: gibbs free energy
- Replies: 5
- Views: 611
Re: gibbs free energy
The Gibbs Free Energy is the energy associated with a reaction that can be used to do work. This is a function of the enthalpy of the reaction minus the temperature multiplied by the entropy. It can be used to see the spontaneity of a reaction, with a negative Gibbs Free energy representing a sponta...
- Wed Feb 13, 2019 10:18 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Problem #4 on Test #1
- Replies: 1
- Views: 237
Re: Problem #4 on Test #1
Kp for any reaction is = [products]/[reactants] but you wouldn't include solids or liquids in this eq constant. Because the reactant is a solid, the only thing that affects Kp is the products. Therefore, Kp = [products] = 0.13. This means the [CO2(g)] = 0.13 atm.
- Wed Feb 13, 2019 10:14 am
- Forum: Calculating Work of Expansion
- Topic: Hotdog Midterm #6
- Replies: 2
- Views: 378
Re: Hotdog Midterm #6
Because work is not a state property, you can't only look at the initial and final state. You would have to add up all the work done in each step of the pathway to get the total work done.
- Wed Feb 13, 2019 10:10 am
- Forum: Thermodynamic Definitions (isochoric/isometric, isothermal, isobaric)
- Topic: Isobaric
- Replies: 12
- Views: 1712
Re: Isobaric
For a constant pressure system, you would be able to use the equation W=-P*deltaV, as the P would be constant and thus can be multiplied by the change in volume to get the work done.
- Sat Feb 09, 2019 2:22 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Hmwrk 11.45 6th edition
- Replies: 3
- Views: 713
Re: Hmwrk 11.45 6th edition
Because this problem is giving you the molar concentrations (given mmol and L), you would use the column Kc, as the book says that the Kc column is the eq constant for the molar concentration of gases.
- Fri Feb 08, 2019 12:56 am
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: Internal energy
- Replies: 1
- Views: 325
Re: Internal energy
Change in internal energy would be 0 for an ideal gas in the case when there is constant temperature (isothermal). Because change in temperature is 0, then change in internal energy is 0, as the two variables are related through the equation: deltaU = 3/2*n*R*deltaT.
- Fri Feb 08, 2019 12:40 am
- Forum: Entropy Changes Due to Changes in Volume and Temperature
- Topic: When C, Heat Capacity is not given?
- Replies: 1
- Views: 188
Re: When C, Heat Capacity is not given?
If you are referencing problem 9.3 in 6th Edition textbook, for part (a), you would use the equation deltaS=deltaQ/T . You would get deltaQ = +65J and T = 25 + 273 = 298 K. This gives you a deltaS of 65/298 = 0.22J/K You would use the same process for part (b), except with 373 K instead of 298. For ...
- Fri Feb 08, 2019 12:34 am
- Forum: Entropy Changes Due to Changes in Volume and Temperature
- Topic: Degeneracy
- Replies: 1
- Views: 253
Re: Degeneracy
Degeneracy is the number of ways of achieving a given energy state. For example, the orbitals in the 2p sublevel are degenerate, as they have the same energy. Degeneracy can be calculated through the equation degeneracy = X^N, where X = possible microstates and N= # of particles.
- Wed Jan 30, 2019 6:04 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Standard Enthalpy of Combustion
- Replies: 1
- Views: 249
Re: Standard Enthalpy of Combustion
I think the solution manual is just giving a visual way of explaining what you did in your head. It is just showing how the combustion reactions cancel out to form the equation given in the exercise and why you would flip the sign for Hc of C2H6. I don't think you would necessarily need to show all ...
- Wed Jan 30, 2019 5:58 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: 6th Edition, 8.57
- Replies: 1
- Views: 282
Re: 6th Edition, 8.57
Hc is the standard enthalpy change of combustion. This means that the given values are the change in enthalpy when each substance reacts with O2, and thus the change in enthalpy when the substances are the reactants in the equation. Because C2H6 is a product in the equation given in the exercise, yo...
- Wed Jan 30, 2019 5:39 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Cp/Cv formulas
- Replies: 2
- Views: 294
Re: Cp/Cv formulas
Cv = (3/2)R and Cp = (5/2)R would be true for any monoatomic ideal gas, so you would use these equations in those cases.
If a molecule is linear, it wouldn't be a monoatomic ideal gas, so you would use the other equations for constant pressure and constant volume.
If a molecule is linear, it wouldn't be a monoatomic ideal gas, so you would use the other equations for constant pressure and constant volume.
- Sat Jan 26, 2019 10:42 pm
- Forum: Entropy Changes Due to Changes in Volume and Temperature
- Topic: Molar Entropy
- Replies: 3
- Views: 322
Re: Molar Entropy
The molar entropy would also increase as a substance goes from solid to liquid to gas.
- Tue Jan 22, 2019 9:52 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Using partial pressure versus molar concentration
- Replies: 2
- Views: 245
Re: Using partial pressure versus molar concentration
Yes, you would be solving for Kp.
- Tue Jan 22, 2019 9:50 pm
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Le Chatelier and water
- Replies: 3
- Views: 1290
Re: Le Chatelier and water
Water in liquid form would not affect the Q or K of a reaction. Because water(l) doesn't affect Q, if you added water to a system at equilibrium, the Q would equal K, meaning the system remains at equilibrium. This is only if water is in liquid form. If it is a gas then you would use that in calcula...
- Wed Jan 16, 2019 12:27 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: 6th edition 11.53
- Replies: 1
- Views: 240
Re: 6th edition 11.53
First, you would find the initial concentration of each substance. For H2, it is 0.4 mol/3L = 0.133M. For I2, it is 1.6mol/3L=0.533M. For HI, it is 0M. Using the balanced chemical reaction, change in H2 is -x, change in I2 is -x, and change in HI is +2x. This means the eq composition of each are: H2...
- Wed Jan 16, 2019 12:14 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Chemical Equilibrium Part 3 Post-Assessment
- Replies: 1
- Views: 250
Re: Chemical Equilibrium Part 3 Post-Assessment
I believe it should just be +2x, not c+2x, because I used eq composition of SO3 to be 2x to solve the problem and it said I got the correct answer. The c may have come from problem 12, where you were asked to find what the value of c would be (where c=+2x)
- Tue Jan 15, 2019 11:25 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Hmwrk 11.45 6th edition
- Replies: 3
- Views: 713
Re: Hmwrk 11.45 6th edition
a) To solve this problem, you would use the reaction Cl2(g) <-> 2Cl(g). From table 2, you see that the K for this reaction is 1.2*10^-7. ICE TABLE: Because it asks for the equilibrium composition, you can use the ICE table (initial, change, equilibrium). To set this up, you would need to find the in...
- Wed Jan 09, 2019 10:27 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Calculating equilibrium concentrations given only Kc?
- Replies: 1
- Views: 189
Re: Calculating equilibrium concentrations given only Kc?
Given only K, you would have to make an ICE table and then use the final equilibrium line from your table in the expression for K (K=[eq line of products]/[eq line of reactant 1][eq line of reactant 2]). Because you are given the K value, you would set the expression equal to the K value of 0.56. Yo...
- Wed Jan 09, 2019 10:14 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: 6th Edition 11.45
- Replies: 1
- Views: 165
Re: 6th Edition 11.45
yes, you should use the same process as (a) but use the value of F2 <-> 2F of 1.2*10^-4. All the other calculations should remain constant. The ICE table would be 0.001-x for F2 and 2x for 2F. This would make the Kc value (2x)^2/(0.0010-x)=1.2*10^-4. Solving for x using the quadratic formula, you wo...
- Wed Jan 09, 2019 9:37 pm
- Forum: Non-Equilibrium Conditions & The Reaction Quotient
- Topic: Chemical Equilibrium Part 2 Question 19
- Replies: 2
- Views: 244
Re: Chemical Equilibrium Part 2 Question 19
First, it says it is in a 3L vessel, so you would divide all the given values by 3 to get the moles per 1 liter, as that is the molarity used in the calculation. Looking at the equation, the As is solid, so you would not need to account for it in the Qc. Solving the problem, you would divide the two...
- Tue Dec 04, 2018 11:27 pm
- Forum: Naming
- Topic: Anionic ligands
- Replies: 5
- Views: 609
Re: Anionic ligands
This is how you would name it under the IUPAC Name Convention. Lavelle uses a different convention, but he says that he accepts both.
There is a sheet on Lavelle's website that has ligands and their names, including the IUPAC Name Convention.
There is a sheet on Lavelle's website that has ligands and their names, including the IUPAC Name Convention.
- Tue Dec 04, 2018 11:24 pm
- Forum: Naming
- Topic: Water as a ligand
- Replies: 5
- Views: 621
Re: Water as a ligand
Yes, you would use aqua. Lavelle has posted a "Naming Coordination Compounds" sheet on his website that has some common ligands and their names.
- Tue Dec 04, 2018 10:48 pm
- Forum: Conjugate Acids & Bases
- Topic: Conjugates
- Replies: 2
- Views: 309
Re: Conjugates
For an acid, the conjugate base would just be the structure of the molecule after the acid donates a proton (when it is deprotonated). For a base, the conjugate acid is when the base accepts the proton (when it is protonated). There is a more detailed description and examples in the textbook on pg 4...
- Fri Nov 30, 2018 3:40 pm
- Forum: Lewis Acids & Bases
- Topic: Mixing up Lewis and Bronsted
- Replies: 3
- Views: 502
Re: Mixing up Lewis and Bronsted
I posted this on another question, I hope this helps! Bronsted looks at protons, while Lewis looks at electron pairs. A Bronsted acid is something that is a proton donor, while a Bronsted base is a proton acceptor. A Lewis acid is an electron acceptor, while a lewis base is an electron donor. see th...
- Fri Nov 30, 2018 2:32 pm
- Forum: Bronsted Acids & Bases
- Topic: Bronsted vs Lewis
- Replies: 3
- Views: 340
Re: Bronsted vs Lewis
Bronsted looks at protons, while Lewis looks at electron pairs. A Bronsted acid is something that is a proton donor, while a Bronsted base is a proton acceptor. A Lewis acid is an electron acceptor, while a lewis base is an electron donor. see this link for examples: http://www.personal.psu.edu/user...
- Fri Nov 30, 2018 2:27 pm
- Forum: Hybridization
- Topic: Determining Factors
- Replies: 3
- Views: 427
Re: Determining Factors
Hybridization is based on the number of electron densities around the atom, so a good rule to follow is that the number of regions of electron density equals the number of hybridization orbitals.
- Sun Nov 25, 2018 12:25 pm
- Forum: Sigma & Pi Bonds
- Topic: Sigma and pi bonds and hybridization
- Replies: 2
- Views: 544
Re: Sigma and pi bonds and hybridization
Molecules that undergo hybridization will still follow the rules we learned before about sigma and pi bonds. For a single bond (ex: C 2sp3 - H 1s in CH4), there would be a single bond between the atoms and thus a sigma bond between the atoms. For a double bond (ex: C double bond C in C2H4), there wo...
- Sun Nov 25, 2018 12:14 pm
- Forum: Dipole Moments
- Topic: *Hydrogen Bonding
- Replies: 2
- Views: 405
Re: *Hydrogen Bonding
In lecture, we were told that it is present in molecules with N,O,or F. We were also told that for a H bond to occur, there needs to be an H atom covalently bound to an electronegative atom and close to another electronegative atom that has an available lone pair. It needs a lone pair because the lo...
- Sun Nov 25, 2018 12:10 pm
- Forum: Interionic and Intermolecular Forces (Ion-Ion, Ion-Dipole, Dipole-Dipole, Dipole-Induced Dipole, Dispersion/Induced Dipole-Induced Dipole/London Forces, Hydrogen Bonding)
- Topic: Difference in Electronegativity
- Replies: 3
- Views: 441
Re: Difference in Electronegativity
I believe we don't need to memorize the exact electronegativity of elements, but that we should know general trends, as this is useful in figuring out types of bonding or polar/nonpolar molecules.
- Sun Nov 18, 2018 9:47 pm
- Forum: Interionic and Intermolecular Forces (Ion-Ion, Ion-Dipole, Dipole-Dipole, Dipole-Induced Dipole, Dispersion/Induced Dipole-Induced Dipole/London Forces, Hydrogen Bonding)
- Topic: Question 6.19 (Sixth Edition)
- Replies: 2
- Views: 1389
Re: Question 6.19 (Sixth Edition)
a) Xenon is larger and has more electrons. This means it will have increased attractive forces, making the melting point higher. b) There will be a higher vapor pressure when the attractive forces are not as strong. Because water has hydrogen bonding, the diethyl ether will have the higher vapor pre...
- Sun Nov 18, 2018 9:33 pm
- Forum: Sigma & Pi Bonds
- Topic: Visualizing sigma and pi bonds
- Replies: 7
- Views: 793
- Sun Nov 18, 2018 9:24 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Types of Bonds
- Replies: 4
- Views: 475
Re: Types of Bonds
sigma bonds have orbitals end to end and allow the bound atoms to rotate. pi bonds have orbitals that overlap side to side and don't allow bound atoms to rotate. a single bond is a sigma bond and a double bond is one sigma one pi bond
- Sun Nov 11, 2018 5:28 pm
- Forum: Lewis Structures
- Topic: Exceptions to Octet Rule
- Replies: 5
- Views: 1036
Re: Exceptions to Octet Rule
Elements in period 3 have an empty d-orbital, so it does not have to obey the octet rule. Hydrogen, helium, beryllium, and lithium are exceptions to the octet rule because they form duplets. Radicals are also exceptions to said rule.
- Sun Nov 11, 2018 5:19 pm
- Forum: Bond Lengths & Energies
- Topic: 3.123 6th ed
- Replies: 1
- Views: 221
Re: 3.123 6th ed
A compound can be radical if it has an unpaired electron.
- Sun Nov 11, 2018 5:13 pm
- Forum: Bond Lengths & Energies
- Topic: finding bond length
- Replies: 6
- Views: 576
Re: finding bond length
Normally bond lengths are given based on experimental data.
- Tue Oct 30, 2018 1:47 pm
- Forum: Formal Charge and Oxidation Numbers
- Topic: 6th edition 3.23
- Replies: 1
- Views: 252
Re: 6th edition 3.23
The oxidation number is determined by the electrons that would be added/lost to form a noble-gas configuration. Because Cl has 7 e-, it would add one electron to get to the electron configuration of Argon. Because it adds one electron, it would have an oxidation number of -1.
- Tue Oct 30, 2018 1:21 pm
- Forum: Electron Configurations for Multi-Electron Atoms
- Topic: Why is it 4f14 if there are 15 elements in that row
- Replies: 2
- Views: 1490
Re: Why is it 4f14 if there are 15 elements in that row
4f14 is the full orbital of elements from lanthanum to Ytterbium (14 elements). Lutetium is the first element in the 5d orbital.
- Tue Oct 30, 2018 1:18 pm
- Forum: Properties of Light
- Topic: Problem A15
- Replies: 2
- Views: 522
Re: Problem A15
You got the correct wavelength, however, you just switched n1 and n2 in the equation. The equation should be: wavelength = Rydberg constant *((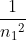 ) - (
) - (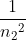 )). If you use this, you should get the correct answer of
)). If you use this, you should get the correct answer of 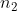 = 3.
= 3.
- Thu Oct 25, 2018 10:15 pm
- Forum: Properties of Light
- Topic: Chapter 1.15 6th Edition
- Replies: 2
- Views: 317
Re: Chapter 1.15 6th Edition
this question says the wavelength is equal to 102.6 nm. This wavelength corresponds to the Lyman series, a set of lines in the UV region of the spectrum with n1 = 1. This is why the answer uses the assumption that n1 = 1. If the wavelength corresponded to the Balmer series, then n1 = 2.
- Thu Oct 25, 2018 10:09 pm
- Forum: Trends in The Periodic Table
- Topic: CH 2 6TH EDITION 2.47
- Replies: 2
- Views: 325
Re: CH 2 6TH EDITION 2.47
Ge: [Ar] 3d10 4s2 4p2, so it would be 4p orbital
Mn: [Ar] 3d5 4s2, so 4s
Ba: [Xe] 6s2, so 6s
Au: [Xe] 4f14 5d10 6s1, so 6s
Mn: [Ar] 3d5 4s2, so 4s
Ba: [Xe] 6s2, so 6s
Au: [Xe] 4f14 5d10 6s1, so 6s
- Thu Oct 25, 2018 5:06 pm
- Forum: Electron Configurations for Multi-Electron Atoms
- Topic: Electron Affinty
- Replies: 2
- Views: 356
Re: Electron Affinty
Electron affinity is the energy released when an electron is added to a gas-phase atom. If the electron affinity is positive, then energy is released when the electron is added to the atom. This would occur if the electron has a lower energy when it occupies the atom's orbital. If the electron affin...
- Wed Oct 17, 2018 10:18 pm
- Forum: Einstein Equation
- Topic: Question 1.13 (Sixth Edition)
- Replies: 1
- Views: 127
Re: Question 1.13 (Sixth Edition)
a) use the Rydberg equation found on page 7 of 6th edition textbook. You already know Rydberg's constant, and you are given n2=4 and n1=2. You just plug these values in and you can get the wavelength. This should be 486 nm. b) The Balmer series consists of lines with n1 = 2 and the Lyman series cons...
- Wed Oct 17, 2018 9:59 pm
- Forum: *Particle in a Box
- Topic: Work Function
- Replies: 3
- Views: 710
Re: Work Function
The work function is the energy required to remove an electron from a metal. In class, it was also referred to as the threshold. In order for an electron to be removed from the metal, the E=h*freq has to be greater than or equal to the work function (threshold energy). (see textbook pages 12-13 for ...
- Wed Oct 17, 2018 9:53 pm
- Forum: Einstein Equation
- Topic: 6th Edition Ch1.33B
- Replies: 1
- Views: 173
Re: 6th Edition Ch1.33B
In part (b), they are asking for the energy that is needed to remove the electron. This means that you would use the equation E=h*frequency. This is why the solution uses the value (2.5x10^16 1/s), as this is the value that is given for frequency. Because you already know h (Planck's constant), you ...
- Sat Oct 13, 2018 9:56 pm
- Forum: Properties of Electrons
- Topic: Quantum World 1.33
- Replies: 4
- Views: 385
Re: Quantum World 1.33
You would want to use the De Broglie equation for this question (wavelength = h/m*v). You would need to look up the mass of the electron and then you are given Planck's constant and the velocity. From there, you would get the wavelength of the ejected electron. Make sure to convert everything to SI ...
- Sat Oct 13, 2018 9:51 pm
- Forum: Properties of Light
- Topic: energy of light
- Replies: 2
- Views: 188
Re: energy of light
for (a), you would use the equations c=(wavelength)*(freq) and E=h*(freq) to derive the equation E=h*c/(wavelength). Because you are given h, c, and wavelength, you can calculate the energy given off by an excited sodium atom. Using this energy calculation, you can answer parts (b) and (c) by conver...
- Sat Oct 13, 2018 7:18 pm
- Forum: Photoelectric Effect
- Topic: Example from Class- Photoelectric Effect
- Replies: 2
- Views: 179
Re: Example from Class- Photoelectric Effect
You would use the equations E=h*(freq) and c=(wavelength)*(freq). From these equations, you derive the equation wavelength=(h*c)/(E). Because you are given E, h, and c, you can then solve for wavelength.
- Sat Oct 06, 2018 11:04 pm
- Forum: Molarity, Solutions, Dilutions
- Topic: Question E.15
- Replies: 1
- Views: 136
Re: Question E.15
There is a mystery metal in this problem, signified by the letter M in M(OH)2. They give you the molar mass of M(OH)2 so that you can get the molar mass of the mystery metal from there. The sulfide of this metal would be the compound that results from the mystery metal bonding with the element sulfi...
- Wed Oct 03, 2018 1:42 pm
- Forum: Empirical & Molecular Formulas
- Topic: Online Resources
- Replies: 2
- Views: 427
Re: Online Resources
There's Khan Academy which has videos and other resources for a lot of different chemistry topics: https://www.khanacademy.org/science/chemistry
- Wed Oct 03, 2018 1:25 pm
- Forum: Empirical & Molecular Formulas
- Topic: problem F13
- Replies: 3
- Views: 386
Re: problem F13
In lecture, Lavelle used the mass composition to provide the mass (g) of an element given a sample of 100 g (ex: given a mass composition of C = 45%, the mass of C would be 45g). Because the problem directly gives you the mass (g), you don't need to calculate the mass composition, as you can already...

