Search found 61 matches
- Mon Mar 11, 2019 4:17 pm
- Forum: Second Order Reactions
- Topic: Final
- Replies: 32
- Views: 2401
Re: Final
It's cumulative, but I would focus more on the newest topics and topics that were not heavily covered on the midterm and two tests.
- Mon Mar 11, 2019 4:16 pm
- Forum: Method of Initial Rates (To Determine n and k)
- Topic: Rate Order Graphs
- Replies: 3
- Views: 475
Re: Rate Order Graphs
i find this diagram pretty helpful in determining order: (shows zero, first, and second order graphs respectively)
- Mon Mar 11, 2019 4:07 pm
- Forum: Zero Order Reactions
- Topic: Graphs
- Replies: 6
- Views: 871
Re: Graphs
here are the graphs for zero order, first order, and second order, respectively
- Tue Mar 05, 2019 10:04 am
- Forum: General Rate Laws
- Topic: Order of Reaction
- Replies: 6
- Views: 690
Re: Order of Reaction
We should be prepared to find, for the purposes of this class, the zeroth order, first order, and second order. There are orders higher than that, but they are rare and we are not expected to do calculations involving those.
- Tue Mar 05, 2019 10:03 am
- Forum: General Rate Laws
- Topic: units of rates and rate constants
- Replies: 4
- Views: 485
Re: units of rates and rate constants
The above responses are correct. You can think of dividing the units by moles as you move up to the next order.
- Tue Mar 05, 2019 9:58 am
- Forum: Kinetics vs. Thermodynamics Controlling a Reaction
- Topic: Study Advice
- Replies: 73
- Views: 7113
Re: Study Advice
I like Lyndon's and Karen's because they have worksheets you can practice.
- Tue Feb 19, 2019 4:48 pm
- Forum: Van't Hoff Equation
- Topic: Equation
- Replies: 4
- Views: 657
Re: Equation
We are not technically using the Van't Hoff Equation, we are using a derivation that is useful for the purposes of this class.
- Tue Feb 19, 2019 4:46 pm
- Forum: Van't Hoff Equation
- Topic: Constants and formulas
- Replies: 7
- Views: 1064
Re: Constants and formulas
We never have to be able to completely derive an equation, only manipulate one and solve it.
- Tue Feb 19, 2019 4:43 pm
- Forum: Van't Hoff Equation
- Topic: Practice Test
- Replies: 5
- Views: 1088
Re: Practice Test
We don't need to know how to derive it, only to manipulate/solve it.
- Sun Feb 17, 2019 11:00 pm
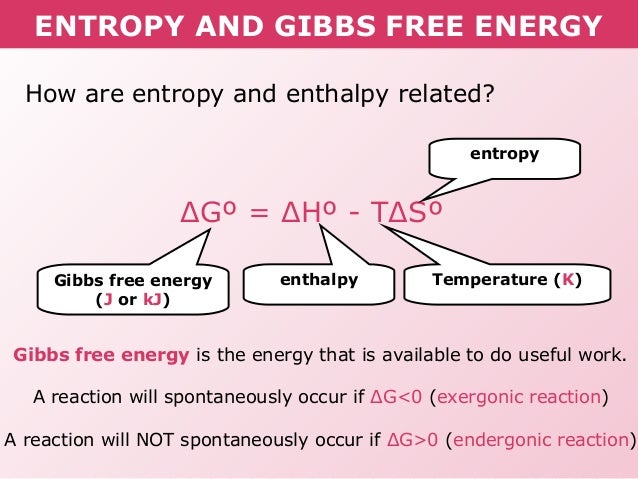
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: calculating delta G
- Replies: 4
- Views: 449
Re: calculating delta G
If given degrees Celsius, convert to K.
- Sun Feb 17, 2019 10:59 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Gibbs Free Energy
- Replies: 7
- Views: 923
Re: Gibbs Free Energy
Eva Guillory 2E wrote:
What do exergonic and endergonic mean?
- Sun Feb 17, 2019 10:57 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Spontaneity
- Replies: 4
- Views: 460
Re: Spontaneity
You know a reaction is spontaneous when delta G is negative. If it's positive, the reaction is not spontaneous.
- Tue Feb 12, 2019 9:44 am
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: Difference between delta U and delta H?
- Replies: 3
- Views: 445
Re: Difference between delta U and delta H?
Delta H is equal to q at constant pressure, whereas delta U=q+w.
- Tue Feb 12, 2019 9:41 am
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: assuming no work
- Replies: 2
- Views: 239
Re: assuming no work
Work is interpreted as energy used in the process of compression or expansion of a system. This means the value of work changes when the volume changes. For this reason, w=0 when there is no compression or expansion.
- Tue Feb 12, 2019 9:37 am
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: answer format
- Replies: 4
- Views: 448
Re: answer format
If they ask for the change in internal energy, give your answer in J or kJ.
- Mon Feb 04, 2019 1:41 pm
- Forum: Phase Changes & Related Calculations
- Topic: Heat Curve
- Replies: 6
- Views: 637
Re: Heat Curve
We will be given the curve if we need the values to solve a problem. Lavelle never makes us memorize constants.
- Mon Feb 04, 2019 1:39 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Bond Enthalpies
- Replies: 4
- Views: 434
Re: Bond Enthalpies
Other than pure visualization, there is no shortcut.
- Mon Feb 04, 2019 1:37 pm
- Forum: Phase Changes & Related Calculations
- Topic: Heat and Condensation
- Replies: 9
- Views: 959
Re: Heat and Condensation
Condensation is exothermic.
- Tue Jan 29, 2019 9:40 am
- Forum: Phase Changes & Related Calculations
- Topic: Phase changes
- Replies: 14
- Views: 1421
Re: Phase changes
I know phase changes from liquid to vapor, solid to liquid, and solid to vapor are always endothermic. Would the opposite phases changes (vapor to liquid, and liquid to solid) always be exothermic? Yes, you are correct. In the first changes you listed, heat acts like a reactant. This means the reac...
- Tue Jan 29, 2019 9:37 am
- Forum: Phase Changes & Related Calculations
- Topic: Steam
- Replies: 11
- Views: 1004
Re: Steam
To answer this question, you must refer to the heating curve. In this case, steam has more energy in Kj/mol, meaning it will burn your hand more upon contact due to the higher amount of energy of vaporized water (compared to the lower amount of energy in liquid water).
- Tue Jan 29, 2019 9:33 am
- Forum: Phase Changes & Related Calculations
- Topic: Standard entalpy of formation
- Replies: 6
- Views: 1051
Re: Standard entalpy of formation
When a molecule is in its most stable state, the standard enthalpy of formation equals 0.
- Wed Jan 23, 2019 12:35 pm
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Endothermic vs. Exothermic
- Replies: 8
- Views: 812
Re: Endothermic vs. Exothermic
When a reaction is endothermic, think of heat as a reactant.
When a reaction is exothermic, think of heat as a product.
When a reaction is exothermic, think of heat as a product.
- Wed Jan 23, 2019 12:31 pm
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Chem Eq. Module #4 Question 15 Conflicting Answers
- Replies: 2
- Views: 331
Re: Chem Eq. Module #4 Question 15 Conflicting Answers
This is similar to when we discuss endothermic or exothermic reactions. If heat is added or removed, the equilibrium shifts. However, heat is not included in K. Same goes for water in this example.
- Wed Jan 23, 2019 12:28 pm
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Chemical Equilibrium part 4 post module assessment question #12
- Replies: 2
- Views: 263
Re: Chemical Equilibrium part 4 post module assessment question #12
This is because the stoichiometric coefficients/ratio are the same on each side, meaning it won't favor either side.
- Mon Jan 21, 2019 6:22 pm
- Forum: Administrative Questions and Class Announcements
- Topic: DOWNLOAD SESSION WORKSHEETS HERE - Sun 7-9PM (Karen)
- Replies: 179
- Views: 23876
Re: DOWNLOAD SESSION WORKSHEETS HERE - Sun 7-9PM (Karen)
Meigan Wu 2E wrote:On Worksheet 2, why is the concentration of CO3 the same as Ka2 for problem 4b?
^ I have the same question
- Wed Jan 16, 2019 12:49 pm
- Forum: Non-Equilibrium Conditions & The Reaction Quotient
- Topic: stability
- Replies: 3
- Views: 385
Re: stability
Stability is based on whether the reaction favors the products. A more stable reaction would favor the products, making the K value larger.
- Wed Jan 16, 2019 12:46 pm
- Forum: Ideal Gases
- Topic: pressure [ENDORSED]
- Replies: 13
- Views: 1112
Re: pressure [ENDORSED]
For the effect of something like pressure, use the ideal gas law PV=nRT. Increasing something on one side will increase something on the other side.
- Wed Jan 16, 2019 12:43 pm
- Forum: Ideal Gases
- Topic: Homework for week 2 [ENDORSED]
- Replies: 10
- Views: 3004
Re: Homework for week 2 [ENDORSED]
Anything that's not too far off base from what we've learned so far is good. This all relates to chemical equilibria.
- Thu Jan 10, 2019 10:12 am
- Forum: Ideal Gases
- Topic: Ideal vs Real Gas
- Replies: 6
- Views: 548
Re: Ideal vs Real Gas
haleyervin7 wrote:For purposes of calculation, do we always assume it is an ideal gas?
For the purposes of this class, we need to assume that a gas is an ideal gas in order to use the idea gas equation. If the problem requires calculations, it's safe to assume it's an ideal gas.
- Thu Jan 10, 2019 10:05 am
- Forum: Ideal Gases
- Topic: Post Assessment
- Replies: 3
- Views: 252
Re: Post Assessment
A similar way to think about it is that if K>10^3, the reaction favors the products. If K<10^3, the reaction favors the reactants.
- Thu Jan 10, 2019 10:03 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Giving Qc or Qp when asked for Q
- Replies: 5
- Views: 629
Re: Giving Qc or Qp when asked for Q
When they give you values in bars, use Qp. When they give you values in M (mol/L), use Qc.
- Mon Dec 03, 2018 1:48 pm
- Forum: Bronsted Acids & Bases
- Topic: Comparing pH levels
- Replies: 3
- Views: 285
Re: Comparing pH levels
Not sure if we have to answer that directly, yet it's good to know for problem solving and checking your answer.
- Mon Dec 03, 2018 1:47 pm
- Forum: Naming
- Topic: Metal Suffix
- Replies: 2
- Views: 484
Re: Metal Suffix
Yes, you only add -ate if the whole compound is negative.
- Mon Dec 03, 2018 1:46 pm
- Forum: Naming
- Topic: Order of Ligand Naming
- Replies: 6
- Views: 575
Re: Order of Ligand Naming
Saman Andalib 1A wrote:You name the ligands in a coordination compound in alphabetical order.
To follow up, is it alphabetical order by prefix or by the names of the ligands?
- Mon Nov 26, 2018 9:21 pm
- Forum: *Molecular Orbital Theory (Bond Order, Diamagnetism, Paramagnetism)
- Topic: test #3
- Replies: 14
- Views: 1536
Re: test #3
Maggie Pickford 3F wrote:So what exactly is paramagnetism and diamagnetism?
Paramagnetic means that there is only one electron in the subshell, whereas diamagnetic means that two electrons are paired (with opposite spin) in the same subshell.
- Mon Nov 26, 2018 9:20 pm
- Forum: *Molecular Orbital Theory (Bond Order, Diamagnetism, Paramagnetism)
- Topic: test #3
- Replies: 14
- Views: 1536
Re: test #3
ariellasarkissian_3H wrote:Also, are we responsible for memorizing the bond angles for each shape?
Yes, he mentioned that you must memorize the VSEPR shapes and their bond angles.
- Mon Nov 26, 2018 9:18 pm
- Forum: Hybridization
- Topic: electron occupying unhybridized orbital
- Replies: 2
- Views: 225
Re: electron occupying unhybridized orbital
This is because a hybridization gives the molecule a lower energy state than if it were unhybridized. This makes it more stable.
- Tue Nov 20, 2018 10:27 am
- Forum: Hybridization
- Topic: Hybridized Orbitals
- Replies: 4
- Views: 402
Re: Hybridized Orbitals
The d orbital exists when there is an expanded octet.
- Tue Nov 20, 2018 10:25 am
- Forum: Hybridization
- Topic: Identifying types of bonds and hybridization in Lewis Structures
- Replies: 3
- Views: 503
Re: Identifying types of bonds and hybridization in Lewis Structures
We are only expected to draw the Lewis structures as we had done so before. He just wants us to be able to identify the geometry (VSEPR model) by looking at the Lewis structure. We are not expected to draw the VSEPR model ourselves.
- Tue Nov 20, 2018 10:21 am
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Bold/Dashed Lines in Bonds
- Replies: 4
- Views: 603
Re: Bold/Dashed Lines in Bonds
It doesn't matter which atoms for which the bonds you make bold or dashed if the bonds are symmetrical with that shape because the structure can rotate. It's just a different view.
- Wed Nov 14, 2018 5:40 pm
- Forum: Dipole Moments
- Topic: dipole distance
- Replies: 3
- Views: 352
Re: dipole distance
Here's a way I like to think about it:
single bond- longest, weakest
double bond
triple bond- shortest, strongest
single bond- longest, weakest
double bond
triple bond- shortest, strongest
- Wed Nov 14, 2018 5:34 pm
- Forum: Electronegativity
- Topic: Electronegativity Values
- Replies: 2
- Views: 306
Re: Electronegativity Values
For reference, there is a figure in the electronegativity section showing the electronegativity values on the periodic table. However, for the test, just know that the further apart elements are on the periodic table, the greater difference in electronegativity they will have.
- Wed Nov 14, 2018 5:31 pm
- Forum: Electronegativity
- Topic: ChemistryHow are electronegativity values for elements determined?
- Replies: 3
- Views: 400
Re: ChemistryHow are electronegativity values for elements determined?
The electronegativity values are experimentally calculated. There is a chart in the book that lists electronegativity values, so you can just use that. As a general rule, elements further apart on the periodic table will have a higher difference in electronegativity.
- Thu Nov 08, 2018 9:45 am
- Forum: Octet Exceptions
- Topic: electron affinity
- Replies: 6
- Views: 682
Re: electron affinity
Fluorine is kind of an exception to the general rule of electron affinity. Typically, you would expect F to have a higher electron affinity since it is higher in the group than Cl. F is very small though, so there is electron-electron repulsion when you add a new electron because there are already e...
- Thu Nov 08, 2018 9:36 am
- Forum: Formal Charge and Oxidation Numbers
- Topic: How do both intersect?
- Replies: 3
- Views: 370
Re: How do both intersect?
Finding the oxidation number of each element in a molecule gives you the formal charge of each element and subsequently that of the whole molecule.
- Thu Nov 08, 2018 9:30 am
- Forum: Resonance Structures
- Topic: Radicals
- Replies: 11
- Views: 1414
Re: Radicals
Radicals have one unpaired, lone valence electron. They are very unstable and don't exist long by themselves in nature.
Here's an example of what it looks like and how you would draw it:
Here's an example of what it looks like and how you would draw it:
- Tue Oct 30, 2018 11:22 pm
- Forum: Formal Charge and Oxidation Numbers
- Topic: Lowest Energy/Stable
- Replies: 2
- Views: 397
Re: Lowest Energy/Stable
Most stable/lowest energy are essentially the same in this context. As for the structures, if you compare two separate structures that have different formal charges assigned, the one with the formal charge closest to zero would be considered most stable/lowest energy.
- Tue Oct 30, 2018 11:18 pm
- Forum: Ionic & Covalent Bonds
- Topic: Interactions between ions
- Replies: 3
- Views: 392
Re: Interactions between ions
We only need to know what he lists on his lecture outlines on his website for 14A. I would check the Chemical Bonds outline!
- Tue Oct 30, 2018 11:14 pm
- Forum: Lewis Structures
- Topic: Exception to Octet Rule [ENDORSED]
- Replies: 3
- Views: 343
Re: Exception to Octet Rule [ENDORSED]
The exceptions start in the second row of the p-block, and continue in the rows of the p-block below that.
- Sat Oct 27, 2018 11:07 pm
- Forum: Ionic & Covalent Bonds
- Topic: Help on 3.39
- Replies: 2
- Views: 370
Re: Help on 3.39
remember that ionic bonds do not share electrons!
- Sat Oct 27, 2018 11:01 pm
- Forum: Electron Configurations for Multi-Electron Atoms
- Topic: Shielding effect
- Replies: 6
- Views: 1187
Re: Shielding effect
For me, it helps to use the bonfire metaphor that Dr. Lavelle mentioned in lecture. Let's say you're an electron sitting close to the fire. If another person (another electron) sits or stands in between you and the fire, then you will feel less heat (or attraction) to the fire (nucleus). In a sense,...
- Sat Oct 27, 2018 9:43 pm
- Forum: Trends in The Periodic Table
- Topic: Homework 2.67b
- Replies: 2
- Views: 256
Re: Homework 2.67b
Additionally, in general the elements in the top right of the periodic table have a higher electron affinity. So by this general guideline, carbon would have a higher electron affinity.
- Sun Oct 21, 2018 2:30 pm
- Forum: Properties of Electrons
- Topic: Quantum World 1.33
- Replies: 4
- Views: 383
Re: Quantum World 1.33
Does anyone know with certainty that we will be given the mass of an electron on the test?
- Sun Oct 21, 2018 2:28 pm
- Forum: Properties of Electrons
- Topic: Wavelength Calculations
- Replies: 5
- Views: 642
Re: Wavelength Calculations
To add to the previous response, we will only be given the equations for E=hv and c=λv, which we must combine to produce E=hc/λ on the test.
- Sun Oct 21, 2018 2:23 pm
- Forum: Properties of Electrons
- Topic: Atomic Spectra: absorption vs emission
- Replies: 3
- Views: 201
Re: Atomic Spectra: absorption vs emission
As far as I know, the electrons must still jump up or down an energy level, but there is the same energy difference between each. So absorption would be +eV, and emission would be the same amount but -eV.
- Sat Oct 13, 2018 5:50 pm
- Forum: Photoelectric Effect
- Topic: Module Question 30 (Part C)
- Replies: 1
- Views: 184
Module Question 30 (Part C)
Light hits a sodium metal surface and the velocity of the ejected electron is 6.61 x 105 m.s-1. The work function for sodium is 150.6 kJ.mol-1. Will someone walk me through this? 30. C. What is the frequency of the incident light on the sodium metal surface? A. 3.01 x 1014 Hz B. 2.27 x 1038 Hz C. 4....
- Sat Oct 13, 2018 5:49 pm
- Forum: Photoelectric Effect
- Topic: Module Question 29 (Part B)
- Replies: 4
- Views: 515
Module Question 29 (Part B)
Light hits a sodium metal surface and the velocity of the ejected electron is 6.61 x 105 m.s-1. The work function for sodium is 150.6 kJ.mol-1. Will someone walk me through this? 29. B. How much energy is required to remove an electron from one sodium atom? A. 2.501 x 10-22 J B. 1.506 x 105 J C. 2.5...
- Sat Oct 13, 2018 5:48 pm
- Forum: Photoelectric Effect
- Topic: Module Question 28 Part A
- Replies: 1
- Views: 163
Module Question 28 Part A
Light hits a sodium metal surface and the velocity of the ejected electron is 6.61 x 105 m.s-1. The work function for sodium is 150.6 kJ.mol-1. Will someone walk me through this? A. What is the kinetic energy of the ejected electron? A. 3.01 x 1025 J B. 3.98 x 10-19 J C. 7.96 x 10-19 J D. 1.99 x 10-...
- Thu Oct 04, 2018 5:32 pm
- Forum: Molarity, Solutions, Dilutions
- Topic: Periodic Table and Molar Mass?
- Replies: 2
- Views: 202
Re: Periodic Table and Molar Mass?
During lecture, Dr. Lavelle mentioned that we would always be given the periodic table on tests (even when we do not need it) so that we can feel comfortable. I am assuming he was referring to not only the midterm and final but the discussion quizzes/tests as well.
- Thu Oct 04, 2018 5:28 pm
- Forum: Balancing Chemical Reactions
- Topic: Net number of molecules
- Replies: 3
- Views: 522
Re: Net number of molecules
1. Add up the coefficients of each side of the equation (this gives you the total amount of moles on each side).
2. Find the difference in moles on each side (subtract the total moles of products from the total moles of reactants).
2. Find the difference in moles on each side (subtract the total moles of products from the total moles of reactants).
- Thu Oct 04, 2018 5:21 pm
- Forum: Accuracy, Precision, Mole, Other Definitions
- Topic: Molecule vs Formula Unit
- Replies: 4
- Views: 833
Re: Molecule vs Formula Unit
Essentially, when they are asking for the amount of molecules, they are referring to an element or something covalently bonded (think Na or O2). When they are asking for the amount of formula molecules, they are referring to an ionic compound (NaCl). You must use Avogadro's number for both situation...