Search found 61 matches
- Sun Mar 17, 2019 4:49 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: Cp vs Cv
- Replies: 6
- Views: 2009
Re: Cp vs Cv
Use Cp when there is constant pressure and Cv when there is constant volume. The problem will specify if it is at constant pressure or volume.
- Sun Mar 17, 2019 4:45 pm
- Forum: First Order Reactions
- Topic: Slopes of a plot
- Replies: 7
- Views: 1229
Re: Slopes of a plot
Since first order is ln[A]=-kt+ln[A]o it has a negative slope due to the -k.
- Sun Mar 17, 2019 4:41 pm
- Forum: Kinetics vs. Thermodynamics Controlling a Reaction
- Topic: Bimolecular
- Replies: 13
- Views: 2259
Re: Bimolecular
Bimolecularity refers to having two molecules colliding in a reaction.
- Sun Mar 17, 2019 4:38 pm
- Forum: Work, Gibbs Free Energy, Cell (Redox) Potentials
- Topic: Wmax
- Replies: 3
- Views: 874
Re: Wmax
To have the greatest Wmax you want the greatest delta G
- Sun Mar 17, 2019 4:35 pm
- Forum: Work, Gibbs Free Energy, Cell (Redox) Potentials
- Topic: Endergonic vs. Endothermic
- Replies: 6
- Views: 1924
Re: Endergonic vs. Endothermic
Endergonic is a positive delta G while endothermic is a positive delta H
- Sun Mar 17, 2019 4:31 pm
- Forum: Phase Changes & Related Calculations
- Topic: heating curves
- Replies: 4
- Views: 1505
Re: heating curves
The heating curve we were introduced to in class was one specific to water. All molecules have their own heating curves
- Sun Mar 17, 2019 4:23 pm
- Forum: Kinetics vs. Thermodynamics Controlling a Reaction
- Topic: intermediate
- Replies: 26
- Views: 2053
Re: intermediate
Intermediates are produced in one elementary step and consumed in the next. They are not part of the overall reaction so they will not appear in the rate law.
- Sun Mar 17, 2019 4:19 pm
- Forum: Arrhenius Equation, Activation Energies, Catalysts
- Topic: Catalysts
- Replies: 28
- Views: 1776
Re: Catalysts
Catalysts lower the activation so it will lower the curve of the reaction.
- Sun Mar 17, 2019 4:16 pm
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Adding an Inert Gas
- Replies: 4
- Views: 615
Re: Adding an Inert Gas
Inert gases do not cause any shift in the system
- Sun Mar 17, 2019 4:14 pm
- Forum: Method of Initial Rates (To Determine n and k)
- Topic: Types of Molecularity
- Replies: 4
- Views: 2261
Re: Types of Molecularity
Unimolecular--1 reactant molecule
Bimolecular--2 reactant molecules
Termolecular--3 reactant molecules
Bimolecular--2 reactant molecules
Termolecular--3 reactant molecules
- Sun Mar 17, 2019 4:11 pm
- Forum: Experimental Details
- Topic: picking a trial
- Replies: 13
- Views: 1614
Re: picking a trial
It does not matter which trial is used, they will all give you the correct value.
- Sun Mar 17, 2019 4:10 pm
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Effects of Compression
- Replies: 5
- Views: 620
Re: Effects of Compression
Yes it would affect the molecules in the gas phase. More specifically, compression increases pressure which will cause the side with less moles to be favored.
- Sun Mar 17, 2019 3:58 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: K and Kc
- Replies: 6
- Views: 964
Re: K and Kc
K and Kc are basically the same thing, except that Kc specifically means concentrations like Kp would refer to partial pressures.
- Sun Mar 17, 2019 2:43 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Q
- Replies: 6
- Views: 854
Re: Q
They do not need to be in standard conditions
- Sun Mar 17, 2019 2:41 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: Work done vs work on system
- Replies: 18
- Views: 3887
Re: Work done vs work on system
Work done on the system is positive while work done by the system is negative
- Sun Mar 17, 2019 2:39 pm
- Forum: Ideal Gases
- Topic: K and Q
- Replies: 8
- Views: 1130
Re: K and Q
You exclude solids and liquids because they remain the same throughout the reaction
- Sun Mar 17, 2019 12:24 am
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Reversible vs irreversible
- Replies: 1
- Views: 434
Reversible vs irreversible
Why does gas expansion in a reversible, isothermal process perform more work than irreversible?
- Sat Mar 16, 2019 9:00 pm
- Forum: Arrhenius Equation, Activation Energies, Catalysts
- Topic: k(1)/k(-1)
- Replies: 4
- Views: 613
Re: k(1)/k(-1)
k is referring to the rate of the forward reaction while k' is referring to the rate of the reverse reaction
- Sat Mar 16, 2019 8:55 pm
- Forum: Administrative Questions and Class Announcements
- Topic: LYNDON'S PORK RAMEN REVIEW
- Replies: 37
- Views: 7568
Re: LYNDON'S PORK RAMEN REVIEW
Why for number ten is it that having a negative standard reaction potential mean that it will be non-spontaneous and therefore the coin will not dissolve?
- Sat Mar 16, 2019 8:52 pm
- Forum: Zero Order Reactions
- Topic: third order
- Replies: 11
- Views: 1222
Re: third order
Possibly, it is in the curriculum.
- Fri Mar 15, 2019 9:14 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: 7th Edition 6N.9
- Replies: 1
- Views: 284
7th Edition 6N.9
A tin electrode in 0.015M Sn(NO 3 ) 2 (aq) is connected to a hydrogen electrode in which the pressure of H 2 is 1.0 bar. If the cell potential is 0.061 V at 25 degrees Celsius, what is the pH of the electrolyte at the hydrogen electrode? How do you set up the cell equation with the given information...
- Fri Mar 15, 2019 6:45 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Adding Pt(s) to cell diagram
- Replies: 5
- Views: 554
Re: Adding Pt(s) to cell diagram
Are there any exceptions to this?
- Fri Mar 15, 2019 6:42 pm
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: 7th edition 6N.3a
- Replies: 1
- Views: 397
7th edition 6N.3a
In the solutions, they kept the partial pressures and concentrations in the calculations and proceeded to solve. Why are the concentrations not converted to partial pressures or vice versa so that all of the values are in the same form?
- Fri Mar 15, 2019 10:07 am
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Adding Pt(s) to cell diagram
- Replies: 5
- Views: 554
Adding Pt(s) to cell diagram
When do we add Pt(s) to a cell diagram?
- Thu Mar 14, 2019 10:06 pm
- Forum: Arrhenius Equation, Activation Energies, Catalysts
- Topic: Activation Energies
- Replies: 2
- Views: 315
Activation Energies
How does the activation energies of reactions help determine if it is endothermic or exothermic? For example, in the 7th edition question 7D.7b asks if the reaction is endothermic or exothermic and the solution talks about the activation energies of the forward and reverse reaction.
- Thu Mar 14, 2019 9:41 pm
- Forum: Arrhenius Equation, Activation Energies, Catalysts
- Topic: 7D.5
- Replies: 1
- Views: 111
7D.5
The rate constant of the reaction between CO 2 and OH - in aqueous solution to give the HCO 3 - ion is 1.5 x 10 10 L.mol -1 s -1 at 25 degrees Celsius. Determine the rate constant at human body temperature (37 degrees Celsius), given that the activation energy for the reaction is 38 kJ.mol -1 . In t...
- Sun Feb 10, 2019 1:58 pm
- Forum: Administrative Questions and Class Announcements
- Topic: UA Sessions Today
- Replies: 2
- Views: 365
Re: UA Sessions Today
These are the scheduled Sunday sessions:
Ajith Raja 11am-1pm (Drop-In), Nathan Mallipeddi 1-3pm (Step-Up), Ryan Cerny 6-7pm (Drop-In), Karen Leung 7-9pm (Workshop), Ryan Cerny 9-10pm (Drop-In)
You can also find this information on Dr. Lavelle's website under "Important Midterm Information"
Ajith Raja 11am-1pm (Drop-In), Nathan Mallipeddi 1-3pm (Step-Up), Ryan Cerny 6-7pm (Drop-In), Karen Leung 7-9pm (Workshop), Ryan Cerny 9-10pm (Drop-In)
You can also find this information on Dr. Lavelle's website under "Important Midterm Information"
- Sun Feb 10, 2019 1:55 pm
- Forum: Thermodynamic Systems (Open, Closed, Isolated)
- Topic: Midterm #1 14B
- Replies: 17
- Views: 2198
Re: Midterm #1 14B
If you search "hotdog" you'll be able to find his post.
- Fri Feb 08, 2019 11:18 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Calculating q
- Replies: 1
- Views: 290
Calculating q
What is the difference between  and
and 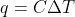 and in which situations would I use them?
and in which situations would I use them?
- Mon Jan 21, 2019 4:32 pm
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: 5J13 7th Ed
- Replies: 2
- Views: 249
Re: 5J13 7th Ed
Looking at the equilibrium constants of each of the reactions at their respective temperatures, the equilibrium constant for the reaction at 700. K is smaller than the constant at 600. K. This tells us that the reactants are favored more in the higher temperature.
- Mon Jan 21, 2019 4:24 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Strong and Weak Acids
- Replies: 3
- Views: 324
Strong and Weak Acids
Why do solutions of weak acids have higher pH values than solutions of strong acids at the
same concentration?
same concentration?
- Sat Dec 08, 2018 10:28 am
- Forum: Octet Exceptions
- Topic: Exceptions
- Replies: 10
- Views: 1377
Re: Exceptions
Exceptions being in the 3rd period since they can expand into their d orbital
- Sat Dec 08, 2018 10:25 am
- Forum: Interionic and Intermolecular Forces (Ion-Ion, Ion-Dipole, Dipole-Dipole, Dipole-Induced Dipole, Dispersion/Induced Dipole-Induced Dipole/London Forces, Hydrogen Bonding)
- Topic: boiling point
- Replies: 5
- Views: 1256
Re: boiling point
When looking at boiling point, you want to examine intermolecular forces. Since NH3 is going to have stronger intermolecular forces (N--H is hydrogen bonding which is the strongest IMF) the boiling point will be greater.
- Sat Dec 08, 2018 10:23 am
- Forum: Properties of Light
- Topic: final Grade
- Replies: 3
- Views: 695
Re: final Grade
We need to know how to name coordination compounds
- Sun Dec 02, 2018 5:05 pm
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: Coordination Compound
- Replies: 1
- Views: 184
Coordination Compound
In the drawing of a coordination compound, some of the compounds were written in reverse to show which atom in that compound interacted with the central atom. How do we know when a different atom interacts with the central atom and to write the compound in reverse?
- Sun Dec 02, 2018 4:44 pm
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: Coordination Number
- Replies: 10
- Views: 961
Re: Coordination Number
Coordination number corresponds to the number of bonded atoms around the central atom
- Sun Dec 02, 2018 4:37 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Dissociation energy
- Replies: 7
- Views: 655
Re: Dissociation energy
Because dissociation energy is an addition of energy to break a bond it is going to be positive.
- Sun Nov 25, 2018 2:06 pm
- Forum: Polarisability of Anions, The Polarizing Power of Cations
- Topic: Polarizability
- Replies: 5
- Views: 454
Polarizability
What does it mean to say that highly distorted electrons are described as being highly polarizable?
- Sun Nov 25, 2018 12:41 pm
- Forum: Dipole Moments
- Topic: canceling dipoles
- Replies: 2
- Views: 420
Re: canceling dipoles
I like to think about a molecules shape to determine if there is symmetry or not in the polar bonds. If so, the dipoles cancel out meaning there is no net dipole.
- Sat Nov 24, 2018 8:38 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: What is VSPER
- Replies: 14
- Views: 1016
Re: What is VSPER
VSEPR stands for "valence shell electron pair repulsion" and we probably do not need to have it memorized
- Fri Nov 16, 2018 8:40 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: AX3E2 Shape
- Replies: 6
- Views: 1226
Re: AX3E2 Shape
A molecule with AX3E2 VSEPR formula is going to have an electron geometry of trigonal bipyramidal (this takes into account the lone pairs) and a molecular geometry of a T-shape (this takes into account only the atoms, excluding the lone pairs).
- Fri Nov 16, 2018 1:14 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Bond Angles
- Replies: 4
- Views: 514
Re: Bond Angles
I don't think it is possible to find an exact figure for the bond angles that are, for instance, less than 120 or 109.5. There are only estimates or a range that it could be in.
- Fri Nov 16, 2018 1:08 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Lone pairs
- Replies: 2
- Views: 208
Re: Lone pairs
I believe the reason is that lone pairs occupy more space, in other words they are more diffused. This causes them to push the other atoms closer together.
- Fri Nov 09, 2018 1:23 pm
- Forum: SI Units, Unit Conversions
- Topic: Angstrom
- Replies: 5
- Views: 962
Re: Angstrom
I talked to my TA about this and even though it isn't an SI unit Dr. Lavelle wants us to know the conversion.
- Fri Nov 09, 2018 1:19 pm
- Forum: Bond Lengths & Energies
- Topic: Strength of Bonds
- Replies: 9
- Views: 1078
Re: Strength of Bonds
^ Yes, the stronger the bond the more energy it will take to break it resulting in a higher boiling point.
- Fri Nov 09, 2018 1:17 pm
- Forum: Bond Lengths & Energies
- Topic: boiling points
- Replies: 4
- Views: 447
Re: boiling points
Compounds with stronger intermolecular forces have higher boiling points because it takes more energy to break their bonds (the energy is given in the form of heat).
- Fri Nov 02, 2018 10:03 pm
- Forum: Lewis Structures
- Topic: Midterm Chemical Formula Names
- Replies: 3
- Views: 389
Re: Midterm Chemical Formula Names
Dr. Lavelle mentioned in lecture today that we do not need to know nomenclature yet.
- Wed Oct 31, 2018 9:59 pm
- Forum: Ionic & Covalent Bonds
- Topic: octet rule
- Replies: 11
- Views: 1113
Re: octet rule
Atoms want to have 8 electrons in their valence shell, so they seek to either gain or loose electrons. Having 8 valence electrons gives that atom a noble gas configuration which makes them the most stable.
- Wed Oct 31, 2018 9:52 pm
- Forum: Lewis Structures
- Topic: lewis acid/base
- Replies: 2
- Views: 217
Re: lewis acid/base
It is a lewis base if it donates its electron pair, and it is a lewis acid if it accepts an electron pair.
- Thu Oct 25, 2018 9:25 pm
- Forum: Properties of Light
- Topic: Electromagnetic Spectrum
- Replies: 5
- Views: 600
Re: Electromagnetic Spectrum
It will most likely be given, but it can be helpful to familiarize yourself with the spectrum to check if your answers are reasonable.
- Thu Oct 25, 2018 9:16 pm
- Forum: Heisenberg Indeterminacy (Uncertainty) Equation
- Topic: Module Question #17
- Replies: 4
- Views: 564
Re: Module Question #17
I thought of it as 1/100 having one sig fig
- Mon Oct 22, 2018 9:40 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Test 2
- Replies: 2
- Views: 340
Re: Test 2
I believe it's going to be more concept heavy than last test, so having a really good understanding all of the topics is going to be important.
- Wed Oct 17, 2018 9:20 pm
- Forum: DeBroglie Equation
- Topic: Wave properties
- Replies: 3
- Views: 327
Re: Wave properties
In class, we used the baseball as an example based off of the De Broglie equation (wavelength = h/p where p=mv). This equation can be used for anything that has a distinct resting mass, as does a baseball. The baseball example in particular shows that as momentum increases wavelength decreases, and ...
- Wed Oct 17, 2018 9:33 am
- Forum: Properties of Light
- Topic: 7th Edition 1A.3
- Replies: 4
- Views: 282
Re: 7th Edition 1A.3
Energy and frequency and directly proportional, because when the frequency increases so does the energy.
- Wed Oct 17, 2018 9:22 am
- Forum: Quantum Numbers and The H-Atom
- Topic: Equation sheet
- Replies: 4
- Views: 522
Re: Equation sheet
On Dr. Lavelle's website there is a link that has the equation sheet he puts on the front of his tests. It's under the course materials section with the title "Constants and Equations." Hope this helps!
- Tue Oct 09, 2018 8:06 pm
- Forum: SI Units, Unit Conversions
- Topic: Conversions
- Replies: 2
- Views: 339
Re: Conversions
I believe it is a metric ton, so the conversion would be 1 t = 1000 kg.
- Tue Oct 09, 2018 8:02 pm
- Forum: Molarity, Solutions, Dilutions
- Topic: Promblem G5, Part C, Seventh Edition
- Replies: 2
- Views: 273
Re: Promblem G5, Part C, Seventh Edition
In part c you are starting with mg instead of mmol. In parts a and b you were able to just use 0.07967 M as a conversion factor because you already started with moles. Part c makes you convert to the mg to g to mol and then mol to L using M. To help me keep the conversions straight, I split this up ...
- Tue Oct 09, 2018 3:27 pm
- Forum: Accuracy, Precision, Mole, Other Definitions
- Topic: E29 Part D
- Replies: 2
- Views: 335
Re: E29 Part D
I believe it is like doing percent composition without multiplying by 100 to get a percent. (percent composition is part mass/whole mass x 100) In this case, part mass/whole mass = fraction The amount of oxygen is (16.00 x 4), the 16.00 being the molar mass and the four comes from the number of mole...
- Thu Oct 04, 2018 2:45 pm
- Forum: Accuracy, Precision, Mole, Other Definitions
- Topic: Avogadro's Number
- Replies: 9
- Views: 1069
Re: Avogadro's Number
Yes, you use Avogadro's number :)
- Tue Oct 02, 2018 9:14 am
- Forum: Empirical & Molecular Formulas
- Topic: Question F11 from 6th edition
- Replies: 3
- Views: 1803
Re: Question F11 from 6th edition
After I divided the three values by the smallest, I found the subscript of Na to be 2.9548, Al to be 1, and F to be 5.910. Since they're very close to a whole number, I just rounded up and got the empirical formula of Na3AlF6. It would be more obvious if the values needed to be multiplied to somethi...
- Mon Oct 01, 2018 8:59 pm
- Forum: General Science Questions
- Topic: Periodic Table
- Replies: 16
- Views: 1664
Periodic Table
How should you round the atomic weights of the elements on the periodic table when using it for calculations and how many decimal places should you go out to?

