Search found 48 matches
- Sun Mar 17, 2019 10:24 am
- Forum: Administrative Questions and Class Announcements
- Topic: DOWNLOAD SESSION WORKSHEETS HERE - Sun 7-9PM (Karen)
- Replies: 179
- Views: 23735
Re: DOWNLOAD SESSION WORKSHEETS HERE - Sun 7-9PM (Karen)
Sorry, can someone explain worksheet 4 number 5 to me? Thank you!
- Sun Mar 17, 2019 10:17 am
- Forum: Administrative Questions and Class Announcements
- Topic: Saying Thank You to Dr. Lavelle
- Replies: 490
- Views: 547573
Re: Saying Thank You to Dr. Lavelle
Thank you Dr. Lavelle for a great two quarters!
- Sat Mar 16, 2019 11:47 am
- Forum: Method of Initial Rates (To Determine n and k)
- Topic: Negative k value
- Replies: 2
- Views: 1093
Re: Negative k value
Oops, I put in the wrong numbers for that problem. But regardless, can there be a negative k value?
- Sat Mar 16, 2019 11:44 am
- Forum: Method of Initial Rates (To Determine n and k)
- Topic: Negative k value
- Replies: 2
- Views: 1093
Negative k value
For 7B.9 part a in the 7th edition, I got that k is -0.17, but the answer key gives it without the negative. Does k always have to be positive, and if so, do I just drop the negative sign and keep the numerical value the same?
- Sat Mar 16, 2019 11:37 am
- Forum: Reaction Mechanisms, Reaction Profiles
- Topic: K'
- Replies: 4
- Views: 535
Re: K'
k' is the rate constant for the reverse reaction. It'll probably be used with the equation K=k/k', where equilibrium constant is the ratio of kforward over kreverse.
- Sat Mar 16, 2019 11:15 am
- Forum: General Rate Laws
- Topic: Explaining 7B.3 c) in 7th edition
- Replies: 2
- Views: 339
Re: Explaining 7B.3 c) in 7th edition
So then unless it's specified otherwise, do we just assume that the initial product concentrations are 0 and the amount we end up with after time passes is how much the reactant concentration decreased by?
- Sat Mar 16, 2019 11:12 am
- Forum: Student Social/Study Group
- Topic: Lyndon's Review Sess
- Replies: 6
- Views: 1116
Re: Lyndon's Review Sess
It should pop up when you type "PORK RAMEN" into the search bar! :)
- Sat Mar 16, 2019 2:20 am
- Forum: Method of Initial Rates (To Determine n and k)
- Topic: 7th edition 7A. 17
- Replies: 3
- Views: 729
Re: 7th edition 7A. 17
Oh ok, so is the answer key just wrong then? (They calculated it in terms of moles but wrote it down as mmol)
- Sat Mar 16, 2019 1:21 am
- Forum: General Rate Laws
- Topic: Problem 7A. 3 7th edition
- Replies: 3
- Views: 478
Re: Problem 7A. 3 7th edition
Is it because we're dealing with rates that the negative isn't present?
- Wed Feb 27, 2019 2:18 pm
- Forum: Administrative Questions and Class Announcements
- Topic: DOWNLOAD SESSION WORKSHEETS HERE - Sun 7-9PM (Karen)
- Replies: 179
- Views: 23735
Re: DOWNLOAD SESSION WORKSHEETS HERE - Sun 7-9PM (Karen)
Samantha Ito 2E wrote:Can someone please explain #4 on the Gibbs Free Energy worksheet?
I used deltaG = deltaGo + RTlnQ. I found Q with the pressure values that were given and assumed temperature to be 25oC, or 298K.
- Wed Feb 27, 2019 2:07 pm
- Forum: Administrative Questions and Class Announcements
- Topic: DOWNLOAD SESSION WORKSHEETS HERE - Sun 7-9PM (Karen)
- Replies: 179
- Views: 23735
Re: DOWNLOAD SESSION WORKSHEETS HERE - Sun 7-9PM (Karen)
Could someone explain #3 on Worksheet 6? How would we find K without using partial pressure? Thanks!
- Tue Jan 29, 2019 3:09 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: enthalpy
- Replies: 4
- Views: 565
Re: enthalpy
State function is a property that depends only on the current state of the system and is independent of how that state was prepared. In other words, it doesn't matter how much or how many times the enthalpy value changed. All that matters is the net result (the sea level example that Dr. Lavelle gav...
- Thu Jan 24, 2019 10:41 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: 7th edition 6D.5
- Replies: 3
- Views: 365
7th edition 6D.5
Calculate the pH, pOH, and percentage protonation of solute in each of the following aqueous solutions: (a) 0.057M NH3(aq); etc.
How do we find the OH- concentration without the Kb given?
How do we find the OH- concentration without the Kb given?
- Wed Jan 16, 2019 7:10 pm
- Forum: Non-Equilibrium Conditions & The Reaction Quotient
- Topic: Percentage Ionization
- Replies: 4
- Views: 516
Re: Percentage Ionization
Percentage deprotonation is the percentage of acid molecules deprotonated.
Percentage protonation is different, since it's the percentage of base molecules protonated.
Percentage protonation is different, since it's the percentage of base molecules protonated.
- Wed Jan 16, 2019 7:06 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: 5I.19 7th edition
- Replies: 1
- Views: 233
5I.19 7th edition
A reaction mixture that consisted of 0.400 mol H 2 and 1.60 mol I 2 was introduced into a flask of volume 3.00 L and heated. At equilibrium, 60.0% of the hydrogen gas had reacted. What is the equilibrium constant K for the reaction H 2 (g) + I 2 (g) <--> 2HI(g) at this temperature? ------------- Sho...
- Tue Jan 15, 2019 1:04 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Change in temperature and K
- Replies: 2
- Views: 295
Re: Change in temperature and K
Think of it like K will determine how pressure/concentration changes (because K is constant, pressure and concentration must increase or decrease to have [product]/[reactant] equal K), while temperature is what determines K.
- Tue Jan 15, 2019 12:53 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Kw
- Replies: 2
- Views: 234
Kw
Will we be assuming that pKw is always 14 for this unit? If we change the temperature to something other than 25 degrees C so that pKw is not 14, won't that mess up the pH and pOH scale?
- Tue Jan 15, 2019 12:46 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Simplifying Cubic Equations
- Replies: 2
- Views: 368
Re: Simplifying Cubic Equations
I don't think any of the textbook questions cover cubic equations, so we'll probably only have to worry about quadratic equations. Not entirely sure though
- Thu Dec 06, 2018 8:21 pm
- Forum: Octet Exceptions
- Topic: Sulfur
- Replies: 3
- Views: 662
Re: Sulfur
Cool, so when in doubt, just check the formal charge. Got it, thank you so much!
- Thu Dec 06, 2018 8:06 pm
- Forum: Octet Exceptions
- Topic: Sulfur
- Replies: 3
- Views: 662
Sulfur
Why does sulfur in SO2 have a normal octet, but sulfate (SO42-) have an expanded octet? Shouldn't it be consistent?
- Thu Dec 06, 2018 7:53 pm
- Forum: Naming
- Topic: Chemical Formulas
- Replies: 2
- Views: 361
Chemical Formulas
Can the transition metal cation ever be outside the brackets of the chemical formula of a coordination compound?
- Thu Dec 06, 2018 7:41 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Intermolecular Forces
- Replies: 1
- Views: 219
Re: Intermolecular Forces
Hope this helps a little!
- Thu Dec 06, 2018 7:35 pm
- Forum: Bond Lengths & Energies
- Topic: bond strength
- Replies: 3
- Views: 799
Re: bond strength
Since boiling point is contingent upon bond strength, which in turn is measured by dissociation energy (energy required to separate bonds completely), the double bonded molecule will have a higher boiling point.
- Thu Dec 06, 2018 7:32 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Lone electron
- Replies: 1
- Views: 269
Re: Lone electron
Yup, molecules with a single lone electron rather than a lone electron pair are radicals. Because of this, radicals tend to be highly reactive and can even damage DNA.
- Thu Dec 06, 2018 7:28 pm
- Forum: Lewis Acids & Bases
- Topic: 6.5
- Replies: 1
- Views: 254
Re: 6.5
The way I thought of it was SO3 can combine with water to form H2SO4, but I'm not sure of the actual reason either. Sorry, hope this helped a little though!
- Thu Dec 06, 2018 7:18 pm
- Forum: Student Social/Study Group
- Topic: Dec 5 Notes
- Replies: 2
- Views: 549
Re: Dec 5 Notes
Thank you so much! :)
- Thu Dec 06, 2018 7:16 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Saying Thank You to Dr. Lavelle
- Replies: 490
- Views: 547573
Re: Saying Thank You to Dr. Lavelle
Dear Dr. Lavelle:
Thank you so much for organizing multiple study sessions to help us out every single week. Chemistry hasn't been the chore I expected it to be, thanks to you! :)
Thank you so much for organizing multiple study sessions to help us out every single week. Chemistry hasn't been the chore I expected it to be, thanks to you! :)
- Thu Dec 06, 2018 3:31 am
- Forum: Student Social/Study Group
- Topic: Dec 5 Notes
- Replies: 2
- Views: 549
Dec 5 Notes
Hi, I came in late to lecture on Wednesday, can someone upload their notes? Sorry, thanks!
- Thu Dec 06, 2018 3:18 am
- Forum: Lewis Acids & Bases
- Topic: HCl vs. HI
- Replies: 7
- Views: 786
Re: HCl vs. HI
I think so, since the key concept is that strong acids lose H + easily, and weaker bonds lead to more acidity. Electronegativity seems to be more relevant for other examples, like hypochlorous acid vs. hypoiodous acid, where Cl's higher electronegativity stabilizes oxygen and makes hypochlorous acid...
- Thu Dec 06, 2018 3:07 am
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Drawing trigonal planar
- Replies: 4
- Views: 907
Drawing trigonal planar
Do we have to draw the trigonal planar diagram with the shaded-in triangle and lined triangle, or would it be fine to just draw a regular Lewis structure with normal lines?
- Thu Dec 06, 2018 2:55 am
- Forum: Properties & Structures of Inorganic & Organic Bases
- Topic: Base Trends
- Replies: 1
- Views: 365
Base Trends
If an acid is more acidic the longer its bond length is, does that mean the converse holds true for bases (the shorter the bond length, the more basic the molecule)?
- Thu Dec 06, 2018 2:42 am
- Forum: Naming
- Topic: Parentheses in Naming
- Replies: 1
- Views: 353
Re: Parentheses in Naming
Hmm, I don't think it should change anything? I haven't seen any other compound written like this before.
- Thu Dec 06, 2018 2:36 am
- Forum: Dipole Moments
- Topic: Dipole moment trends
- Replies: 2
- Views: 268
Dipole moment trends
If a molecule has a dipole moment, would that make it more easily dissolvable in water or less? What about melting/boiling temperature?
- Sat Nov 03, 2018 10:54 pm
- Forum: Ionic & Covalent Bonds
- Topic: Chem Midterm
- Replies: 13
- Views: 1171
Re: Chem Midterm
8 questions, some with multiple parts to them! :)
- Sat Nov 03, 2018 10:53 pm
- Forum: Ionic & Covalent Bonds
- Topic: Uncertainty Principles
- Replies: 2
- Views: 328
Re: Uncertainty Principles
Think of uncertainty like a range: if the velocity given is 373.23 plus or minus .34 m/s, the velocity will be something between 372.89 m/s and 373.57 m/s. We know it must fall between these two values but we're not sure what the exact velocity will be, so the uncertainty is 373.57-372.89 = 0.68. Lo...
- Sat Nov 03, 2018 10:45 pm
- Forum: Quantum Numbers and The H-Atom
- Topic: Determining Amount of Electrons From Quantum Numbers
- Replies: 4
- Views: 6003
Re: Determining Amount of Electrons From Quantum Numbers
Unless the problem were specific about what element it is looking for or how many electrons are in the outmost subshell (ex: 3d 2 ), it would probably be too vague to give a clear answer. Perhaps you could give a range of how many electrons there could potentially be? For example, if the only inform...
- Sun Oct 28, 2018 3:45 pm
- Forum: Ionic & Covalent Bonds
- Topic: Hydrogen Bonds
- Replies: 5
- Views: 498
Re: Hydrogen Bonds
Since there is no actual electron exchange taking place (whether by the sharing of electrons in covalent bonds or the transfer of electrons in ionic bonds), hydrogen bonds are much weaker, as they only rely electromagnetic attraction.
- Sun Oct 28, 2018 3:39 pm
- Forum: Trends in The Periodic Table
- Topic: E- affinity and electronegativity [ENDORSED]
- Replies: 1
- Views: 339
Re: E- affinity and electronegativity [ENDORSED]
When there is a high effective nuclear charge, more energy is released when the electrons are attached; this accounts for the high electron affinity in the top right of the periodic table. Electronegativity is tangentially related to this, in that it increases when the effective nuclear charge incre...
- Sun Oct 28, 2018 3:19 pm
- Forum: *Shrodinger Equation
- Topic: H(psi)=E(psi) Equation
- Replies: 2
- Views: 994
Re: H(psi)=E(psi) Equation
I don't think we'll be using it in this course, but if there were to be a problem that makes use of this equation, it'll probably provide the kinetic energy and potential energy and using the hamiltonian found from those two values, you'd be able to calculate the wavefunction and the energy. Because...
- Fri Oct 05, 2018 1:11 am
- Forum: Empirical & Molecular Formulas
- Topic: Empirical formula from MPC
- Replies: 3
- Views: 243
Empirical formula from MPC
When we're determining the empirical formula from the mass percentage composition, would the molar ratio be the the ratio found when we divide all the moles by the smallest mole, or the ratio with the whole numbers? Or does it even matter? Ex: Is molar ratio 1.00 : 1.33 : 1.00 or 3 : 4 : 3? Thank yo...
- Fri Oct 05, 2018 1:07 am
- Forum: SI Units, Unit Conversions
- Topic: Fundamentals Problem E15
- Replies: 4
- Views: 357
Re: Fundamentals Problem E15
Oh okay! That was helpful, thank you! :)
- Tue Oct 02, 2018 7:27 pm
- Forum: Empirical & Molecular Formulas
- Topic: Empirical Formula
- Replies: 5
- Views: 354
Re: Empirical Formula
The best way of approaching this is to try to multiply the decimals by the smallest number possible. For example, we can either multiply based on the 0.13 or the 2.23. 0.13 is close to 0.10, so we would theoretically multiply all the numbers by 10. 2.23 is close to 2.25, so we would theoretically mu...
- Tue Oct 02, 2018 7:11 pm
- Forum: Accuracy, Precision, Mole, Other Definitions
- Topic: number of moles, molecules, and formula units?
- Replies: 3
- Views: 387
Re: number of moles, molecules, and formula units?
Atoms, molecules, and formula units all fall under the same category of actual tangible units. There physically exist atoms, molecules, and formula units (which are basically ionic compounds). However, mole is a hypothetical unit that exists to help us compare the mass of various elements. For each ...
- Tue Oct 02, 2018 7:07 pm
- Forum: SI Units, Unit Conversions
- Topic: Fundamentals Problem E15
- Replies: 4
- Views: 357
Fundamentals Problem E15
The molar mass of the metal hydroxide M(OH)2 is 74.10 g/mol. What is the molar mass of the sulfide of this metal?
Please help! :( I'm not sure where the sulfide is coming from. I don't quite remember what metal hydroxide has to do with sulfide.
Thank you! :)
Please help! :( I'm not sure where the sulfide is coming from. I don't quite remember what metal hydroxide has to do with sulfide.
Thank you! :)
- Sun Sep 30, 2018 10:08 am
- Forum: Molarity, Solutions, Dilutions
- Topic: Study Module Post-assessment Question
- Replies: 5
- Views: 372
Re: Study Module Post-assessment Question
Oh, okay! Thank you! :)
- Sat Sep 29, 2018 1:48 pm
- Forum: SI Units, Unit Conversions
- Topic: Addressing Hydrates in Conversion Problems
- Replies: 1
- Views: 164
Re: Addressing Hydrates in Conversion Problems
Yes, it does include that part. The answer given in the back of the book is 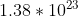 atoms of oxygen, which you get when you assume that there are 11 oxygen atoms in the compound (4 in the sulfate, 7 in the heptahydrate).
atoms of oxygen, which you get when you assume that there are 11 oxygen atoms in the compound (4 in the sulfate, 7 in the heptahydrate).
- Sat Sep 29, 2018 1:30 pm
- Forum: Significant Figures
- Topic: Significant Figures
- Replies: 10
- Views: 2883
Re: Significant Figures
You can use however many significant figures you want for the molar mass and whatever other numbers are needed in the calculation, but eventually, your final answer has to have the same number of significant figures as the numbers provided in the problem.
- Sat Sep 29, 2018 1:22 pm
- Forum: Molarity, Solutions, Dilutions
- Topic: Study Module Post-assessment Question
- Replies: 5
- Views: 372
Study Module Post-assessment Question
I don't know what I did wrong for one of the study module questions for "Molarity and Dilution of a Solution": 5.00 g of KMnO 4 is dissolved in a 150.00 mL flask of water. If 20.00 mL of this solution is removed and placed in a new 2nd 250.00 mL flask and filled with water, what is the con...

