Search found 73 matches
- Fri Mar 15, 2019 9:45 pm
- Forum: General Rate Laws
- Topic: 15.19 6th edition
- Replies: 4
- Views: 434
Re: 15.19 6th edition
I think it's because for each concentration, you need to convert the mmol to mol, because the number gets smaller as you square, and multiply. And it won't get you the same answer if you just convert from mol to mmol at the end, so you have to convert before then.
- Fri Mar 15, 2019 7:48 pm
- Forum: Arrhenius Equation, Activation Energies, Catalysts
- Topic: 15.61 (6th addition)
- Replies: 3
- Views: 359
Re: 15.61 (6th addition)
You have to use the natural log of the Arrhenius equation: ln (K) = lnA - Ea/R (1/T). Then if you subtract, you can get the form: ln(K2/K1) = Ea/R (1/T2 - 1/T1). From this, you can now plug in the given values to get 39 kj per mol Can you explain how to get the second form please? I'm having a diff...
- Fri Mar 15, 2019 2:07 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Cell Diagrams: Solids in different phases
- Replies: 1
- Views: 218
Cell Diagrams: Solids in different phases
So to clarify from what Dr. Lavelle said today during the review session, can it be said that in cell diagrams, if there are multiple solids on one side, they will always be separated by "|" and never by a comma? Or can this only be applied when one solid is an electrode, and the other is ...
- Thu Mar 14, 2019 9:51 am
- Forum: Student Social/Study Group
- Topic: Curve?
- Replies: 50
- Views: 6331
Re: Curve?
allisoncarr1i wrote:Does that line in the syllabus also mean that anyone who receives over 50% of the points will pass?
Yes, this means that as long as students get 50% (250/500 points), they will pass the class with a C- (:
- Thu Mar 14, 2019 9:46 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Midterm Q3
- Replies: 4
- Views: 849
Re: Midterm Q3
So the K_{a2} is the second deprotonation of phosphoric acid, which means two H^{+} ions will be gone. The first deprotonation ( K_{a1} ) would be: H_{3}PO_{4} (aq) + H_{2}O (l) \rightleftharpoons H_{2}PO_{4}^{-} (aq) + H_{3}O^{+} (aq) The second deprotonation ( K_{a2...
- Thu Mar 14, 2019 9:37 am
- Forum: First Order Reactions
- Topic: Writing First Order Reactions
- Replies: 4
- Views: 730
Re: Writing First Order Reactions
He mentioned in class that you usually don't write the "1" since it's already implied, just like how in a math equation where x is to the first power, we write it as "x" instead of "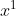 ".
".
- Thu Mar 14, 2019 9:35 am
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: Nernst Equation
- Replies: 2
- Views: 499
Re: Nernst Equation
I believe they will both give you similar answers, the second equation with a number in it is just the simplified version, where the (RT/F) is already calculated, given that the reaction is at the standard temperature 298 K.
Can anyone confirm this?
Can anyone confirm this?
- Thu Mar 14, 2019 9:31 am
- Forum: *Enzyme Kinetics
- Topic: true statement?
- Replies: 4
- Views: 770
Re: true statement?
Yes. When you look at the Arrhenius equation we can see that increasing temperature makes the rate larger, as does lowering the activation energy, which is what a catalyst does, so doing these together should theoretically increase the enhancement of the rate. But isn't the statement saying that th...
- Tue Mar 12, 2019 10:48 pm
- Forum: Reaction Mechanisms, Reaction Profiles
- Topic: Does reaction rate mean average reaction rate?
- Replies: 3
- Views: 488
Does reaction rate mean average reaction rate?
I just wanted to ask, when the book or the final asks you to find the reaction rate for a substance, which reaction rate (average, instantaneous, or unique) do we presume to use?
- Thu Feb 28, 2019 5:14 pm
- Forum: Work, Gibbs Free Energy, Cell (Redox) Potentials
- Topic: Value of n
- Replies: 7
- Views: 963
Re: Value of n
Sometimes there are cases where you simplify the balanced redox reaction, would n be the number of moles before simplification or would it be the number of moles after simplification? In a redox reaction, you would look at the half-reactions you used to cancel out the electrons before you formed th...
- Wed Feb 27, 2019 10:07 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: 6th edition 14.13 d [ENDORSED]
- Replies: 5
- Views: 506
Re: 6th edition 14.13 d [ENDORSED]
I understand that Au (s) is serving as a conducting electrode in this case, but isn't the oxidation reaction that's occurring in this problem Au+(aq) --> Au3+ (aq) + 2e- ? Is it not necessary to include Au+ when writing out the cell diagram for the anode side? You base off your cell diagram from th...
- Wed Feb 27, 2019 8:05 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: H2O in Cell Diagrams
- Replies: 4
- Views: 411
H2O in Cell Diagrams
Is it right to say that 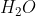 (l) is never included in cell diagrams, but
(l) is never included in cell diagrams, but 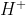 ions and
ions and 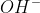 ions are included?
ions are included?
- Wed Feb 27, 2019 7:58 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: delta G naught when K<1
- Replies: 3
- Views: 3128
Re: delta G naught when K<1
Since K is the concentration of products over the concentration of reactants ( \frac{[P]}{[R]} ) at equilibrium, when K<1, this means there is a higher concentration of reactants compared to products. This means that the *reverse* reaction is favored, which in turn means the forward reaction is NOT ...
- Wed Feb 27, 2019 3:18 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Final Topics
- Replies: 6
- Views: 681
Re: Final Topics
I believe the final is cumulative, and I think there might be some basic things we would have to remember from 14A, such as figuring out the limiting reagent given a reaction.
I don't know exactly what else we would be expected to know though:/
I don't know exactly what else we would be expected to know though:/
- Wed Feb 27, 2019 3:15 pm
- Forum: Balancing Redox Reactions
- Topic: Oxidation states for Oxygen
- Replies: 2
- Views: 349
Re: Oxidation states for Oxygen
And after that, you would add the appropriate number of electrons to cancel out the positive Hydrogen ions?
I think I understand now, thank you(:
I think I understand now, thank you(:
- Tue Feb 26, 2019 6:34 pm
- Forum: Balancing Redox Reactions
- Topic: Oxidation states for Oxygen
- Replies: 2
- Views: 349
Oxidation states for Oxygen
How would you figure out what the change in oxidation state is for a reaction where  \rightarrow O_{2} (g)) ? There's no charge there so I could only assume the oxidation states are both 0, but that would not make sense.
? There's no charge there so I could only assume the oxidation states are both 0, but that would not make sense.
- Wed Feb 20, 2019 10:42 pm
- Forum: Ideal Gases
- Topic: atm vs. bar?
- Replies: 25
- Views: 2945
Re: atm vs. bar?
Xingzheng Sun 2K wrote:So which one is the right unit for the equation PV=nRT?
I'd say it depends on which unit the problem uses. Can anyone confirm this?
- Wed Feb 20, 2019 10:40 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: When does delta U equal zero?
- Replies: 17
- Views: 8314
Re: When does delta U equal zero?
amogha_koka3I wrote:Do we assume isothermal, reversible, and at equilibrium are all synonymous in this class?
I believe that we can assume (for this class) that isothermal also means reversible. I don't think we can assume it's at equilibrium though.
- Wed Feb 20, 2019 10:34 pm
- Forum: General Science Questions
- Topic: Midterm
- Replies: 5
- Views: 863
Re: Midterm
Does anyone know how you would've figured out the oxidation states of the reactants and products involved?
- Wed Feb 20, 2019 10:32 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Delta G a state function?
- Replies: 23
- Views: 2835
Re: Delta G a state function?
Aidan Ryan 1B wrote:What are things we have learned that are not state functions?
Basically anything that isn't part of "PD TV HUGS" are not state functions.
Examples of things we've learned now that are NOT state functions would be: work (w), heat (q), and cell potential (E)
- Wed Feb 20, 2019 10:28 pm
- Forum: Ideal Gases
- Topic: units
- Replies: 15
- Views: 1373
Re: units
Remember that K is a constant, and therefore has no units(:
- Wed Feb 20, 2019 10:18 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Midterm Ice Table
- Replies: 6
- Views: 698
Re: Midterm Ice Table
I made the mistake of using an ICE table when we didn't need to use one. Because of that, I also had to approximate because there ended up being a cubic equation if I didn't. But it was not the right method to answer the question:(
- Thu Feb 07, 2019 9:41 pm
- Forum: Administrative Questions and Class Announcements
- Topic: DOWNLOAD SESSION WORKSHEETS HERE - Sun 7-9PM (Karen)
- Replies: 179
- Views: 23723
Re: DOWNLOAD SESSION WORKSHEETS HERE - Sun 7-9PM (Karen)
005199302 wrote:for #6, shouldn't the work be -12.4kJ?
w=-PxdV = (-122atm/L)(101.33J/atmL) = -12.4kJ
Were you able to solve 6a? I've tried doing it but keep getting 12.85 kJ, but I don't see where it went wrong.
- Wed Feb 06, 2019 7:08 pm
- Forum: Administrative Questions and Class Announcements
- Topic: DOWNLOAD SESSION WORKSHEETS HERE - Sun 7-9PM (Karen)
- Replies: 179
- Views: 23723
Re: DOWNLOAD SESSION WORKSHEETS HERE - Sun 7-9PM (Karen)
For Worksheet 1 question 7, are the sig figs correct? Wouldn't it be 3 sig figs, not 4, because the lowest number given is 298 K? Or is it 4 sig figs because we don't use the temperature to solve this problem?
- Thu Jan 31, 2019 11:10 am
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Question on yesterday’s lecture
- Replies: 2
- Views: 320
Re: Question on yesterday’s lecture
That's a good question! I originally thought it was all in Chapter 8 of the 6th edition, but there are some concepts that I can't find. So, if it isn't in Chapter 8, I guess it would be in Chapter 9.
- Thu Jan 31, 2019 11:07 am
- Forum: Phase Changes & Related Calculations
- Topic: What Does U and q stand for?
- Replies: 9
- Views: 2360
Re: What Does U and q stand for?
- Thu Jan 31, 2019 11:02 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: increasing pressure, what happens to concentration?
- Replies: 10
- Views: 2572
Re: increasing pressure, what happens to concentration?
You can imagine that when pressure increases, the gas molecules on both sides of the reaction move faster, and are therefore more likely to bump into each other. The side with more moles of gas will have more molecules that bump into each other, which will drive the reaction to shift towards the sid...
- Thu Jan 31, 2019 10:49 am
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Cv vs. Cp [ENDORSED]
- Replies: 13
- Views: 11866
Re: Cv vs. Cp [ENDORSED]
Kenan Kherallah 2C wrote:Whats R?
I believe R refers to the universal gas constant!
- Thu Jan 24, 2019 12:37 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Assuming K
- Replies: 2
- Views: 353
Assuming K
Let's say there's a question that asks you if a chemical reaction is at equilibrium, and it gives you a reaction and K-value but doesn't specify if the K is Kc or Kp. Then, it gives you the partial pressure concentrations of the reactants and products. Is it safe to assume then, that the K-value giv...
- Wed Jan 23, 2019 9:24 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Initial Q
- Replies: 3
- Views: 460
Re: Initial Q
I am terrible at explaining, but here is the video I watched: https://www.youtube.com/watch?v=54n1XppP-lA and I got confused with the example at 25:46 when the initial concentrations of the products were not 0. The reason why the initial concentrations of the products are not 0, is because they giv...
- Wed Jan 23, 2019 9:17 pm
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Increase in Volume Effect on Equilibrium
- Replies: 6
- Views: 1544
Increase in Volume Effect on Equilibrium
I understand that when you decrease volume, the side with more moles becomes more crowded and "bumps into each other" more often, which is why the reaction then favors the side with fewer moles. But can someone help me visualize why when you increase volume, the side with more moles is fav...
- Tue Jan 22, 2019 9:45 pm
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Le Chatelier and water
- Replies: 3
- Views: 1287
Re: Le Chatelier and water
Since we don't include liquids or solids in K-values, adding water won't change the K-value.The only thing that would affect it is if the concentrations of the reactants or products are increased/decreased, but if the concentrations remain the same, adding water won't affect it.
- Tue Jan 22, 2019 9:38 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Does [H3O+][OH-] always equal 10^-14?
- Replies: 9
- Views: 1213
Re: Does [H3O+][OH-] always equal 10^-14?
I'm not sure what that problem is since I have the 6th edition, but I believe it is 10^-14 under lab conditions, which means the temperature is constant. Even then, I'm not sure if it is ALWAYS equal to 10^-14. I'd like to know too, it's a good question!
- Tue Jan 15, 2019 11:05 pm
- Forum: General Science Questions
- Topic: Rounding/ Significant Figures
- Replies: 3
- Views: 687
Re: Rounding/ Significant Figures
Remember that when looking at a problem, you use the number with the least amount of sig figs for your answer. So in the problem you're talking about, are you sure there wasn't a number given somewhere that only had 1 significant figure? At least from the problems I've looked at, the solution manual...
- Fri Jan 11, 2019 4:20 pm
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Adding a liquid
- Replies: 7
- Views: 561
Re: Adding a liquid
I believe that adding a liquid will not affect the K because it will stay the same on both the reactants and products sides.
- Fri Jan 11, 2019 1:28 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: What do you do if all products are pure substances?
- Replies: 2
- Views: 181
Re: What do you do if all products are pure substances?
Yes, you would only include the gases in the 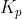 , and the numerator would be 1 since the equilibrium constant for partial pressure is the concentrations of partial pressure of products over partial pressure of reactants:
, and the numerator would be 1 since the equilibrium constant for partial pressure is the concentrations of partial pressure of products over partial pressure of reactants:

- Thu Jan 10, 2019 4:56 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: 6th edition, 11.7?
- Replies: 4
- Views: 342
Re: 6th edition, 11.7?
Thanks that's really helpful. Just wanted to know why would 5/17 be used for the calculation of partial pressure instead of 6/11 from the earlier part B? It's because you're looking for the equilibrium constant K, so you use the flask that's at equilibrium! The flask that's before the one with 5/17...
- Sat Dec 08, 2018 8:00 pm
- Forum: Naming
- Topic: Ligand Names
- Replies: 4
- Views: 643
Re: Ligand Names
I think using either will be fine! I have heard before that Lavelle will be using the old convention. Can someone confirm this please?
- Thu Dec 06, 2018 10:11 pm
- Forum: Bronsted Acids & Bases
- Topic: donating or accepting proton
- Replies: 2
- Views: 342
Re: donating or accepting proton
Yes, I think that's a good way to think about it! The H+ counts as a proton.
- Thu Dec 06, 2018 10:09 pm
- Forum: Polarisability of Anions, The Polarizing Power of Cations
- Topic: Polarizing Power: Size vs. Charge
- Replies: 4
- Views: 722
Re: Polarizing Power: Size vs. Charge
It helps me to know what the trend of sizes are for cations. They generally decrease across a period, and increase down a group (due to the addition of another shell). From there, you can tell that the smaller the cation, the greater its polarizing power. Hope that helps(:
- Thu Dec 06, 2018 9:57 pm
- Forum: Naming
- Topic: Naming with bis-
- Replies: 5
- Views: 565
Re: Naming with bis-
what were the other names we had to remember besides bis when the other prefixes are already used in the name? We need to remember to use: bis- instead of di- tris- instead of tri- tetrakis- instead of tetra- pentakis- instead of penta- We use those names when the ligand already has di-, tri-, or t...
- Fri Nov 30, 2018 5:44 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Pentagonal bipyramidal
- Replies: 4
- Views: 1327
Re: Pentagonal bipyramidal
My TA told us that usually a central atom will at most form 6 bonds, with a total of 12 valence electrons in its outermost shell, so since pentagonal bipyramidal has 7 bonds to its central atom, I think it is a rarity.
- Tue Nov 27, 2018 4:14 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Test 3 Content
- Replies: 3
- Views: 460
Re: Test 3 Content
Rebeca_Ruiz_3D wrote:So just to be clear the test won’t cover anything from Monday’s lecture? Like coordination compounds?
Yes, the test will not cover anything from Monday's lecture!
- Tue Nov 27, 2018 1:29 pm
- Forum: Hybridization
- Topic: hybridization
- Replies: 3
- Views: 443
Re: hybridization
sp and sp2 and so on are the general hybridization of atoms. When it says 2sp2 that refers to the second period orbitals. For example, if we are asked to label the bond between C and H in CH4, we would label it as \sigma (C2sp^{3}, H1s) . This is because Carbon has valence electrons in perio...
- Tue Nov 27, 2018 1:18 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: polarity vs. nonpolarity
- Replies: 8
- Views: 680
Re: polarity vs. nonpolarity
You do have to be careful when looking at dipole moments because some nonpolar molecules can have dipole moments, they just cancel out against other dipole moments. For a molecule to be polar it must have polar bonds with dipoles that don't cancel. Professor Lavelle also said it would be helpful to...
- Tue Nov 27, 2018 11:00 am
- Forum: Sigma & Pi Bonds
- Topic: Sigma and Pi bonds
- Replies: 5
- Views: 416
Re: Sigma and Pi bonds
They could also ask us to label all existing bonds in a molecule. For example, in 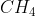 we would label the bond
we would label the bond 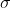 (C2
(C2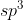 , H1s).
, H1s).
- Tue Nov 27, 2018 10:09 am
- Forum: Ionic & Covalent Bonds
- Topic: Covalent character and Electronegativity
- Replies: 2
- Views: 365
Re: Covalent character and Electronegativity
According to electronegativity trends, Cs has a lower electronegativity value than K, so the difference between Cs and Cl is greater than the EN difference between K and Cl. In this case though, the difference is so great that both KCl and CsCl will be ionic, with CsCl having greater ionic characte...
- Mon Nov 26, 2018 11:52 pm
- Forum: Ionic & Covalent Bonds
- Topic: Covalent character and Electronegativity
- Replies: 2
- Views: 365
Covalent character and Electronegativity
How does electronegativity relate to greater covalent character when comparing 2 molecules?
For example, between KCl and CsCl, which one has the greater covalent character? And why? Does electronegativity explain it?
For example, between KCl and CsCl, which one has the greater covalent character? And why? Does electronegativity explain it?
- Mon Nov 19, 2018 9:47 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: VSPER Formula
- Replies: 7
- Views: 826
Re: VSPER Formula
The VSEPR formula refers to AX(sub n)E(sub m), where A is the central atom, X is the atoms attached to the central atom (so n would be the number of atoms attached), and E refers to electron lone pairs (so m would be the number of lone pairs attached to the central atom). For example, in H20, the VS...
- Sat Nov 17, 2018 12:22 am
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Octahedral
- Replies: 4
- Views: 544
Re: Octahedral
Do the bond angles of the octahedral shape (90 degrees) ever change (because of things like electron pairs)? I believe if there is only one electron pair (AX5E), it will slightly push the other 4 bonded atoms further away from it, so that the angle between the central atom, the opposite atom from t...
- Thu Nov 15, 2018 12:07 am
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Drawing structures
- Replies: 4
- Views: 385
Re: Drawing structures
Yes, if you were to draw the VSEPR model of NH3, given that it has a lone pair, you would draw N as the central atom with a lone electron pair attached, one H with a regular line, one H with the stripes, and one H with the triangular wedge! The striped lines means the H atom is seen in the back, whi...
- Wed Nov 14, 2018 11:59 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Determining Bond Angles
- Replies: 1
- Views: 231
Re: Determining Bond Angles
I think knowing their 3-Dimensional geometry definitely helps determine their bond angles! I would generally say though, that the most common angles are 90, 109.5,120, and 180. It's usually either those angles, or slightly less than those angles (when lone pairs are involved). Thinking about what th...
- Fri Nov 09, 2018 9:14 pm
- Forum: Ionic & Covalent Bonds
- Topic: Ionization Energies
- Replies: 13
- Views: 3680
Re: Ionization Energies
How would you determine these trends for diagonal (non-adjacent) elements? For example, between Carbon and Sulfur. I think between those two, carbon would have a higher ionization energy because sulfur is in period 3, which means it has another shell around the nucleus. The extra shell shields vale...
- Fri Nov 09, 2018 8:33 pm
- Forum: Electronegativity
- Topic: Electronegativity Difference from 1.5-2
- Replies: 1
- Views: 496
Electronegativity Difference from 1.5-2
I know the general rule for categorizing ionic vs covalent bonds, is knowing the difference in electronegativities of the atoms involved, and if it's less than 1.5 it's covalent, and if it's greater than 2 it's ionic. What exactly is the bonding type, or what happens between the atoms, when the elec...
- Fri Nov 09, 2018 4:49 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Tetrahedral Shape Question
- Replies: 3
- Views: 380
Re: Tetrahedral Shape Question
Yes! Because electrons in the real world are 3-dimensional, the electrons maximize their repulsions to each other, and do their best to have as much space between each other as possible. This results in the tetrahedral shape Dr. Lavelle mentioned in class. As for drawing it out, I'm not too sure, bu...
- Wed Oct 31, 2018 8:04 pm
- Forum: Ionic & Covalent Bonds
- Topic: Problem 3.5
- Replies: 1
- Views: 149
Re: Problem 3.5
The ground state electron configuration for Cu would be [Ar]4s^1 3d^10. You would think it would be [Ar]4s^2 3d^9, but a full d-orbital is more favorable than a full s-orbital. This means one electron in the s-orbital will jump to the d-orbital in order for that orbital to be full. So in Cu+, the el...
- Wed Oct 31, 2018 7:55 pm
- Forum: Electron Configurations for Multi-Electron Atoms
- Topic: Electron removal from orbitals question
- Replies: 3
- Views: 383
Re: Electron removal from orbitals question
When you remove an electron from an atom that has a p-orbital (and no d-orbital), you remove it first from p and then from s. However, if you remove an electron from an atom that has a d-orbital, you remove it first from the s-orbital.
- Wed Oct 31, 2018 7:34 pm
- Forum: Trends in The Periodic Table
- Topic: Electron affinity
- Replies: 5
- Views: 339
Re: Electron affinity
I was also very confused by the book definition, but my TA explained electron affinity by saying it's basically how badly an atom wants an electron. The atoms that need only a few more electrons to have a full shell, want electrons much more than those that need many more electrons to fill in their ...
- Thu Oct 25, 2018 12:25 am
- Forum: DeBroglie Equation
- Topic: De Broglie module #35
- Replies: 3
- Views: 418
Re: De Broglie module #35
I think it's true that technically all matter has wavelike properties, but I believe in one of his lectures, Mr. Lavelle mentioned that anything smaller than 10^-18 would be so small that it most likely would not display wavelike properties.
- Thu Oct 25, 2018 12:21 am
- Forum: Heisenberg Indeterminacy (Uncertainty) Equation
- Topic: Indeterminacy Question
- Replies: 3
- Views: 315
Re: Indeterminacy Question
Yes, I would think so! Since the equation involves a greater than or equal to sign, it would make sense that they ask for minimum of something, and not maximum.
- Thu Oct 25, 2018 12:15 am
- Forum: Administrative Questions and Class Announcements
- Topic: Answers to module questions?
- Replies: 2
- Views: 282
Re: Answers to module questions?
If you answer all the questions on the post-modules, the whole thing is scored, and although it doesn't tell you what the correct answers are (for the ones you got wrong), it's still useful to know! And there's always the option of retaking it and choosing different answers until you get 100% of the...
- Sun Oct 21, 2018 12:41 pm
- Forum: Properties of Light
- Topic: HW 1.3 (6th edition)
- Replies: 1
- Views: 197
HW 1.3 (6th edition)
Question 1.3 asks, "What happens when frequency of electromagnetic radiation decreases?" and the answer is "the extent of the change in the electrical field at a given point decreases." Would someone be able to explain to me what the answer to this means, or how this relationship...
- Sun Oct 21, 2018 11:45 am
- Forum: Properties of Light
- Topic: Equations
- Replies: 6
- Views: 639
Re: Equations
Yes, for every test we will be given a sheet of equations and the periodic table!
- Fri Oct 19, 2018 12:05 am
- Forum: *Black Body Radiation
- Topic: Black Body
- Replies: 10
- Views: 1347
Re: Black Body
I believe he mentioned in class that we don't need to know about black bodies, so I wouldn't worry too much about studying them. It's also not on the course outline!
- Tue Oct 16, 2018 10:37 pm
- Forum: Bohr Frequency Condition, H-Atom , Atomic Spectroscopy
- Topic: Question 1.15 sixth edition
- Replies: 10
- Views: 838
Re: Question 1.15 sixth edition
why is the energy level of 102.6 nm 1? My reasoning for why n1=1, is that the 102.6 nm is a part of the Lyman series, and a general rule in the Lyman series is the first energy level (n1) is always equal to 1, so n1=1. I'm not sure if that line where 102.6 nm is is n1, but that was just my reasonin...
- Sat Oct 13, 2018 11:25 pm
- Forum: Properties of Light
- Topic: Units for a Joule [ENDORSED]
- Replies: 3
- Views: 559
Re: Units for a Joule [ENDORSED]
A "Joule" is a unit of energy, and is named after the man who discovered it. I think we just use the term "joule" as a shorter way to say 1 kg*m^2/s^2, and it lets people know automatically that we're referring to energy. I hope that helps at least a bit haha(: !
- Sat Oct 13, 2018 10:31 pm
- Forum: Balancing Chemical Reactions
- Topic: Test 1 and Future Tests
- Replies: 5
- Views: 781
Re: Test 1 and Future Tests
What I end up doing to show my work for balancing equations, is count the number of moles on each side and write them out. For example, if there are 12 total carbon atoms on both sides, I'd write C: 12 on each side! I hoped it would show them that I checked my work.
- Sat Oct 13, 2018 10:14 pm
- Forum: Properties of Light
- Topic: HW 1.7A
- Replies: 3
- Views: 1269
Re: HW 1.7A
I got the same answer of 4.23 x 10^-7, and since nanometers is in 10^-9, I just moved over the decimal so that it would be 10^-9. The answer then becomes 423 x 10^-9, which is the same thing as 423 nanometers. I hope this helps!(:
- Sat Oct 06, 2018 11:41 pm
- Forum: Molarity, Solutions, Dilutions
- Topic: G.13
- Replies: 1
- Views: 136
Re: G.13
You're right, it should be 4 L of water as the final volume! Did you check to see if the final answer is correct? Maybe it was a typo, or they really did solve it incorrectly. The answer should be 1.0 x 10^-2 mol of N!
- Sat Oct 06, 2018 1:10 am
- Forum: Administrative Questions and Class Announcements
- Topic: Number of questions on test one [ENDORSED]
- Replies: 39
- Views: 21672
Re: Number of questions on test one [ENDORSED]
I attended a chemistry study session where I was told there would only be about 3-4 questions, but most likely with multiple parts. I was also told that really studying and understanding what is listed in Outline 1 would help us on the test! I don't know how strict they'll be on grading though:/
- Wed Oct 03, 2018 10:00 pm
- Forum: Significant Figures
- Topic: E23C Sig Figs
- Replies: 2
- Views: 236
Re: E23C Sig Figs
In general, you use the given information to figure out how many sig figs you need in your answer. So yes, in this case you base it off the 25.2 kg in the question, which means your answer will also include 3 sig figs!
- Wed Oct 03, 2018 9:55 pm
- Forum: Empirical & Molecular Formulas
- Topic: Homework
- Replies: 2
- Views: 335
Re: Homework
My chem TA told us to include our Bruin ID number and our discussion session! I'm not sure if that applies to all classes though.
- Tue Oct 02, 2018 9:50 pm
- Forum: Balancing Chemical Reactions
- Topic: Balancing equations (Problem H21 6th edition)
- Replies: 3
- Views: 505
Balancing equations (Problem H21 6th edition)
In this equation, we need to balance the equation C10H15N + O2 -----> CO2 + H2O + CH4N2O, and the answer to that is 2 C10H15N + 26 O2 -----> 19 CO2 + 13 H2O + CH4N2O. I ended up with the correct answer, but it took me a while to figure it out. Does anyone know a faster way to balance out equations, ...

