Search found 66 matches
- Tue Mar 12, 2019 11:48 am
- Forum: General Rate Laws
- Topic: Decreasing Instantaneous Rate
- Replies: 2
- Views: 280
Re: Decreasing Instantaneous Rate
As the concentrations of the reactants decreases, the rate will decrease since rate is dependent on reactant concentration
- Tue Mar 12, 2019 11:47 am
- Forum: First Order Reactions
- Topic: Rate dependency
- Replies: 5
- Views: 561
Re: Rate dependency
We are looking at the limiting reactant, or the one that reacts more slowly
- Tue Mar 12, 2019 11:46 am
- Forum: First Order Reactions
- Topic: Integrated rate law confusion
- Replies: 5
- Views: 494
Re: Integrated rate law confusion
It just depends which side of the equation you isolate. If you move the ln expressions to the right, then you will end up with the ln[A0] on top
- Tue Mar 12, 2019 11:44 am
- Forum: First Order Reactions
- Topic: reaction orders
- Replies: 3
- Views: 372
Re: reaction orders
The order of a reaction is determined by adding up all the exponents in the rate law.
- Wed Mar 06, 2019 10:38 am
- Forum: General Rate Laws
- Topic: unique rate of reaction
- Replies: 2
- Views: 342
Re: unique rate of reaction
You can use this to find the rate of decomposition or formation of the reactants and products by dividing or multiplying their molar coefficients respectively.
- Wed Mar 06, 2019 10:36 am
- Forum: Kinetics vs. Thermodynamics Controlling a Reaction
- Topic: 15.7 or finding rates of decomposition
- Replies: 2
- Views: 297
Re: 15.7 or finding rates of decomposition
You need to know the unique rate of reaction, and from there you divide by the molar coefficient of the molecule you are finding the decomposition rate of.
- Wed Mar 06, 2019 10:35 am
- Forum: Kinetics vs. Thermodynamics Controlling a Reaction
- Topic: kinetics and thermodynamics
- Replies: 4
- Views: 577
Re: kinetics and thermodynamics
Thermodynamics can never tell us the rate of a reaction. often times we use a combination of thermodynamics and kinetics to fully understand a reaction.
- Wed Feb 27, 2019 10:41 am
- Forum: Work, Gibbs Free Energy, Cell (Redox) Potentials
- Topic: flipping the sign of Ecell
- Replies: 5
- Views: 693
Re: flipping the sign of Ecell
This is because E cell is the reduction potential. If you are looking at an oxidation reaction, you have to flip the sign of the reduction potential
- Tue Feb 26, 2019 10:11 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Platinum
- Replies: 2
- Views: 246
Re: Platinum
If the reduction or oxidation reaction does not include a solid, then use platinum
- Thu Feb 21, 2019 11:42 am
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Spontaneity in terms of Gibbs Free Energy
- Replies: 2
- Views: 317
Re: Spontaneity in terms of Gibbs Free Energy
You set the gibbs free energy equation delta H - T x delta S equal to 0. In other words, delta G is 0 because this is the value at which the reaction will change from non spontaneous to spontaneous. If a higher value of the resulting temperature gives a negative Delta G, then the reaction will be sp...
- Thu Feb 21, 2019 11:38 am
- Forum: Van't Hoff Equation
- Topic: ΔSº and ΔHº question
- Replies: 5
- Views: 690
Re: ΔSº and ΔHº question
The change in delta S and delta H is negligible with a change in temperature so we assume they are constant.
- Thu Feb 21, 2019 11:37 am
- Forum: Van't Hoff Equation
- Topic: When Delta S is constant
- Replies: 1
- Views: 301
Re: When Delta S is constant
For a change in temperature when we are looking at the value of k, the change in delta S is negligible, so we don't account for it.
- Wed Feb 20, 2019 3:38 pm
- Forum: Van't Hoff Equation
- Topic: Non-consistant enthalpy
- Replies: 3
- Views: 414
Re: Non-consistant enthalpy
We can assume they are constant because changes in temperature cause so little change in delta H and delta S
- Wed Feb 20, 2019 3:37 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Gibbs and concepts
- Replies: 3
- Views: 364
Re: Gibbs and concepts
You should probably know the values at which delta H and delta S will create a spontaneous reaction
- Wed Feb 20, 2019 3:35 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Delta G
- Replies: 3
- Views: 326
Re: Delta G
A negative delta H means that it is exothermic, and usually exothermic processes are favored because they don't require extra energy
- Wed Feb 20, 2019 3:34 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Spontaneity in the Gibbs Eqn
- Replies: 4
- Views: 450
Re: Spontaneity in the Gibbs Eqn
Just look at the equation delta G=delta H - T delta S. If you plug in the numbers and delta G is negative, then it is spontaneous.
- Tue Feb 12, 2019 3:01 pm
- Forum: Entropy Changes Due to Changes in Volume and Temperature
- Topic: Edition 7 number 4F11
- Replies: 2
- Views: 249
Edition 7 number 4F11
"Q4A. During the test of an internal combustion engine, 3.00L of nitrogen gas at 18.5 degrees C was compressed suddenly (and irreversibly) to .500L by driving in a piston. In the process the temperature of the gas increased to 28.1 degrees C. Assume ideal behavior and 1.00 mole of nitrogen gas....
- Mon Feb 11, 2019 9:13 pm
- Forum: Thermodynamic Systems (Open, Closed, Isolated)
- Topic: difference in states
- Replies: 6
- Views: 772
Re: difference in states
All you really need to pay attention to is whether the pressure, volume, or temperature is constant.
- Fri Feb 08, 2019 12:11 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Limiting Reagents
- Replies: 5
- Views: 653
Re: Limiting Reagents
If you ever are given two reactants that form a product and the answer to the question is dependent on how much the reaction proceeds, you should calculate the limiting reagent.
- Fri Feb 08, 2019 12:10 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Bomb calorimeters vs polystyrene cup calorimeters
- Replies: 3
- Views: 612
Re: Bomb calorimeters vs polystyrene cup calorimeters
The polystyrene calorimeter is an open system. heat can leave. But a bomb calorimeter is closed and isolated
- Fri Feb 08, 2019 12:09 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Knowing which one to use
- Replies: 3
- Views: 606
Re: Knowing which one to use
You just have to read the problem and consider the variables that are being kept constant. If volume is constant, use Cv and if pressure is constant, use Cp and so on. specific heat capacity and molar heat capacity are the same. It should say in the question whether youre dealing with moles or grams.
- Fri Feb 08, 2019 12:07 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Bond
- Replies: 2
- Views: 310
Re: Bond
I dont remember Dr. Lavelle mentioning anything about bond angles
- Fri Feb 08, 2019 12:05 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: q=deltaH under constant pressure
- Replies: 1
- Views: 201
Re: q=deltaH under constant pressure
delta h = the change in internal energy + P delta V. The change in internal energy also equals q + w, and w equals -P delta V. so, at constant pressure and volume, the -P delta V and + P delta V cancel out, leaving you with just delta H = q.
- Mon Jan 28, 2019 8:57 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Heat Capacity
- Replies: 5
- Views: 497
Re: Heat Capacity
Usually they'll give you units for everything so if there are grams in the units then use specific heat capacity and if there are moles use molar heat capacity
- Mon Jan 28, 2019 8:55 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Standard State
- Replies: 2
- Views: 268
Re: Standard State
for the elements, you can look at the periodic table. usually it says what state each element is in at 25 degrees celsius and 1 atm.
- Mon Jan 28, 2019 8:53 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: K versus C*
- Replies: 2
- Views: 239
Re: K versus C*
To convert from Celsius to Kelvin you simply add 273. Since specific heat is the amount of heat required to raise something 1 degree celsius, it is the same as raising it one Kelvin because the difference between 1 degree and 2 degrees celsius is the same as raising it from 274 to 275 degrees Kelvin...
- Mon Jan 21, 2019 8:55 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Quadratic Formula
- Replies: 2
- Views: 307
Re: Quadratic Formula
If you are dealing with a very weak acid or base, then you can assume that x is negligible and eliminate the x from the denominator of your Ka equations, leaving you with x^2/the original concentration of the acid or base. To check if it was correct to assume that x was negligible, check the percent...
- Mon Jan 21, 2019 8:52 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: solving for pka and ka and ph
- Replies: 2
- Views: 188
Re: solving for pka and ka and ph
14-pH equals the pOH
- Mon Jan 21, 2019 8:51 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Ka1 Ka2
- Replies: 3
- Views: 521
Re: Ka1 Ka2
I think that Ka2 is always smaller than Ka1 and you have to do each dissociation as its own problem, using Ka1 for the first and Ka2 for the second dissociation.
- Mon Jan 21, 2019 8:45 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Ka3
- Replies: 2
- Views: 222
Re: Ka3
Dr. Lavelle never went over this in lecture as far as I can remember so I don't think we need to worry about that.
- Mon Jan 14, 2019 9:25 am
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Exothermic Reactions
- Replies: 9
- Views: 847
Re: Exothermic Reactions
In lecture, Dr. Lavelle explained that adding heat to an endothermic reaction will favor the formation of products and adding heat to an exothermic reaction will favor the formation of the reactants.
- Mon Jan 14, 2019 9:22 am
- Forum: Non-Equilibrium Conditions & The Reaction Quotient
- Topic: K and Q [ENDORSED]
- Replies: 7
- Views: 697
Re: K and Q [ENDORSED]
you can find out whether a reaction is at equilibrium using Q if you are given K. Calculate Q, and if the number is equal to K, then the reaction is at equilibrium.
- Mon Jan 14, 2019 9:20 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Change in Concentration
- Replies: 2
- Views: 263
Re: Change in Concentration
if you change the concentrations of the reactants or products, the reaction will balance itself back to equilibrium, and the final concentrations of the reactants and products will yield the equilibrium constants if put into the equation.
- Fri Dec 07, 2018 9:28 pm
- Forum: Calculating pH or pOH for Strong & Weak Acids & Bases
- Topic: SO THERE ARE SPECIAL RULES FOR SIG FIGS IN pH THEN
- Replies: 5
- Views: 804
Re: SO THERE ARE SPECIAL RULES FOR SIG FIGS IN pH THEN
Also, if you are calculating the reverse like 10^1.558, then the answer would be 36.1 because you only use how many sig figs are after the decimal in the exponent
- Fri Dec 07, 2018 12:52 pm
- Forum: Naming
- Topic: Naming Coordination Compounds
- Replies: 3
- Views: 386
Re: Naming Coordination Compounds
You add up the charges of all the ligands and the ions outside of the brackets. for example, if that number adds up to 2- and the charge of the total molecule is 0, then the charge of the metal is 2+.
- Fri Dec 07, 2018 12:48 pm
- Forum: Bronsted Acids & Bases
- Topic: 6th edition J.1
- Replies: 2
- Views: 442
Re: 6th edition J.1
For part a, for example, NH3 is a base because it has a lone pair of electrons on the Nitrogen which wants to accept an proton. HBr is a bronzed base because it wants to donate its hydrogen. KOH will dissociate and accept a proton and turn water into OH-. Im not sure if I am explaining this correctl...
- Fri Dec 07, 2018 12:41 pm
- Forum: Bronsted Acids & Bases
- Topic: Alkali vs Alkaline
- Replies: 2
- Views: 492
Re: Alkali vs Alkaline
Alkali metals are in group 1 and alkaline earth metals are in group 2. Both of them form base compounds. For example NaOH and KOH are from the alkali metals and CaO and Mg(OH)2 are from the alkaline earth metals.
- Sun Dec 02, 2018 3:44 pm
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: Oxidation number
- Replies: 3
- Views: 475
Re: Oxidation number
basically, if you have a transition metal as your central atom and you need to find its oxidation state, then you add up the oxidation numbers of all the atoms attached to it and subtract it from the total charge of the molecule.
- Fri Nov 30, 2018 11:32 am
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Lone Pairs
- Replies: 11
- Views: 956
Re: Lone Pairs
A lone pairs repel a bonding pair more than a bonding pair repels a bonding pair. Two lone pairs have the highest level of repulsion, then a lone pair and a bonded pair, and two bonded pairs have the lowest level of repulsion.
- Fri Nov 30, 2018 11:30 am
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Axial and equatorial atoms
- Replies: 2
- Views: 510
Re: Axial and equatorial atoms
Axial atoms are the atoms that form a 180 degree angle. For example, in a trigonal bipyramidal molecule, the axial atoms would be the two atoms that form the points of the pyramid. In this example, the equatorial atoms would be the ones that form the base triangle at the equator of the axis.
- Fri Nov 30, 2018 11:28 am
- Forum: Properties & Structures of Inorganic & Organic Bases
- Topic: Weak Bases with Nitrogen
- Replies: 4
- Views: 787
Re: Weak Bases with Nitrogen
Dr. Lavelle said that if. a molecule has Nitrogen in it and that Nitrogen has a lone pair then it acts as a base. It is like in a DNA molecule where the nitrogen in the base pair gives up its lone pair.
- Sun Nov 25, 2018 4:05 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: pi and sigma bonds
- Replies: 9
- Views: 1447
Re: pi and sigma bonds
Can someone explain pi and sigma bonds and how to determine which is which?
- Sun Nov 25, 2018 4:03 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Polar molecules
- Replies: 3
- Views: 307
Re: Polar molecules
A good example of this is water. Without the lone pair on the Oxygen atom, the molecule would be nonpolar. However, the extra electrons give the molecule a bent shape, making it polar.
- Sun Nov 25, 2018 4:02 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Test 3
- Replies: 38
- Views: 2668
Re: Test 3
I think so. Everything we've learned after the material that was on the midterm.
- Sun Nov 18, 2018 10:42 am
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Bond Angles
- Replies: 3
- Views: 500
Re: Bond Angles
To determine bond angles you have to first determine the shape of the molecule by drawing the lewis structure and looking at how many bonding pairs and how many lone pairs of electrons it has. From there, you can determine the angle based on the shape and the position that each atom is in.
- Sun Nov 18, 2018 10:40 am
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Drawing structures
- Replies: 8
- Views: 809
Re: Drawing structures
Dr. Lavelle said that we wouldn't be drawing the models with wedges, so I don't think you have to worry about that.
- Mon Nov 12, 2018 6:43 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Molecular Shape Affects Strength of Interaction
- Replies: 3
- Views: 210
Re: Molecular Shape Affects Strength of Interaction
it depends on how big the molecules are and how far apart the polar ends of each molecule are. For example, in dipole-dipole interactions, two spheres will have a weaker interaction because the positive end of one will be further from the negative end of the other. But, with two rod shaped molecules...
- Fri Nov 09, 2018 11:47 am
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Bond Angles
- Replies: 13
- Views: 2357
Re: Bond Angles
Bond angles are determined by the largest angle between to atoms in a molecule. It is dependent on how many bonds are in the molecule. For example, a molecule with two bonds coming off of the central atom will be linear, as a 180 degree angle is the largest possible angle between these two bonds.
- Fri Nov 09, 2018 11:45 am
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Different Shapes
- Replies: 4
- Views: 370
Re: Different Shapes
Dr. Lavelle started going over this in lecture today. The shape of a molecule is always dependent on bonds being as far away from each other as possible. For a molecule with a central atom that has two bonds, it will be linear because the bonds will be farthest apart at 180 degrees. For a molecule w...
- Mon Nov 05, 2018 12:53 pm
- Forum: Lewis Structures
- Topic: C7 in 7th Edition HW
- Replies: 1
- Views: 204
Re: C7 in 7th Edition HW
usually the lone pairs get attached to the central atom which, in this case, is iodine.
- Sun Nov 04, 2018 12:07 pm
- Forum: Lewis Structures
- Topic: Lewis Structures
- Replies: 11
- Views: 1043
Re: Lewis Structures
It is whichever atom is the least electronegative or has the lowest ionization energy.
- Sat Nov 03, 2018 12:26 pm
- Forum: Properties of Electrons
- Topic: electron energy levels
- Replies: 1
- Views: 483
electron energy levels
Hi! I was doing one of the homework problems (1A.15 in edition 7) and the question asks you to find the values of n for the initial and final energy levels of an electron where a line is formed in the UV spectrum at 102.6 nm and energy is emitted. The answer says that the initial n is 1 and the fina...
- Thu Nov 01, 2018 5:06 pm
- Forum: Lewis Structures
- Topic: central atoms
- Replies: 8
- Views: 791
Re: central atoms
Ionization energy and electronegativity follow the same trends on the periodic table, so it doesn't matter which one you use to determine the central atom. It should be the least electronegative and the one with the lowest ionization energy.
- Thu Nov 01, 2018 5:03 pm
- Forum: Lewis Structures
- Topic: Determining Lewis Structure
- Replies: 3
- Views: 261
Re: Determining Lewis Structure
A molecule is most stable when the formal charges all all the atoms in the molecule are zero. The formal charges of all the atoms will also always add up to the total charge of the molecule. Another rule is that if the formal charge of an atom in a molecule has to be negative, make sure the most ele...
- Thu Nov 01, 2018 4:58 pm
- Forum: Lewis Structures
- Topic: Expanded octets
- Replies: 6
- Views: 661
Re: Expanded octets
The most common ones are Sulfur and Phosphorous.
- Wed Oct 24, 2018 4:18 pm
- Forum: Properties of Electrons
- Topic: Electrons and wavelengths?
- Replies: 3
- Views: 213
Re: Electrons and wavelengths?
I'm assuming you could use the equation 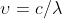
- Wed Oct 24, 2018 4:16 pm
- Forum: Properties of Electrons
- Topic: spectroscopy
- Replies: 1
- Views: 363
spectroscopy
What is the difference between atomic and molecular spectroscopy?
- Wed Oct 24, 2018 4:09 pm
- Forum: Properties of Electrons
- Topic: Electrons and wavelengths?
- Replies: 3
- Views: 213
Re: Electrons and wavelengths?
It is possible for electrons to have wavelengths and frequencies. The De Broglie equation is used to calculate the wavelength of particles (including electrons), and if something has a wavelength, it will also have a frequency by nature.
- Tue Oct 23, 2018 8:30 am
- Forum: Properties of Electrons
- Topic: Orbitals
- Replies: 5
- Views: 797
Re: Orbitals
This is just something you have to memorize. The s orbitals only have one shell, P orbitals have 3, d orbitals have 5 shells, and f orbitals have 7.Each shell holds 2 electrons.
- Tue Oct 23, 2018 8:23 am
- Forum: Properties of Light
- Topic: worksheet3 question11
- Replies: 3
- Views: 369
Re: worksheet3 question11
For part d, higher frequency light has higher energy, so the energy of the photons in that light may be higher than the work function, giving the electrons emitted a higher energy. It is shown by the equation Kinetic Energy of electron=Energy of photon-Work Function. However, not all high frequencie...
- Wed Oct 17, 2018 4:01 pm
- Forum: Properties of Light
- Topic: Electromagnetic Radiation
- Replies: 3
- Views: 353
Re: Electromagnetic Radiation
I think it is because electromagnetic radiation is essential energy going through a space, and when frequency decreases, so will energy since frequency and energy are directly proportional by the equation E=hv
- Wed Oct 17, 2018 3:54 pm
- Forum: Properties of Light
- Topic: Homework Problem
- Replies: 2
- Views: 262
Homework Problem
In the UV spectrum of atomic hydrogen, a line is observed at 102.6 nm. Determine the values of n for the initial and final energy levels of the electron during the emission of energy that leads to this spectral line. This is 1A.15 in the seventh edition textbook, how do you do this?
- Tue Oct 16, 2018 4:06 pm
- Forum: Properties of Light
- Topic: Threshold Energy
- Replies: 4
- Views: 192
Re: Threshold Energy
Ok thank you!
- Mon Oct 15, 2018 3:37 pm
- Forum: Properties of Light
- Topic: Threshold Energy
- Replies: 4
- Views: 192
Threshold Energy
What happens to the electron if the energy of the photon is exactly the same as the work function? Is it still removed?
- Tue Oct 02, 2018 12:02 pm
- Forum: Properties of Light
- Topic: Finding Energy required to remove an electron from one metal atom
- Replies: 3
- Views: 735
Re: Finding Energy required to remove an electron from one metal atom
I calculated the energy of the electron using the formula 1/2mv^2 and I got 1.99x10^-19 J which the answer key says is correct. I thought that by "the energy required to remove an electron from one sodium atom" the question meant the threshold energy, so why isn't the answer 150.6 kJ.mol-1...
- Mon Oct 01, 2018 5:12 pm
- Forum: Properties of Light
- Topic: Finding Energy required to remove an electron from one metal atom
- Replies: 3
- Views: 735
Finding Energy required to remove an electron from one metal atom
Light hits a sodium metal surface and the velocity of the ejected electron is 6.61 x 105 m.s-1. The work function for sodium is 150.6 kJ.mol-1 How much energy is required to remove an electron from one sodium atom? I saw this on the photoelectric effect module and thought I did it correctly, but my ...

