Search found 59 matches
- Sun Mar 17, 2019 11:02 am
- Forum: Arrhenius Equation, Activation Energies, Catalysts
- Topic: Arrhenius equation
- Replies: 2
- Views: 579
Re: Arrhenius equation
Being given the activation energy is a good hint to use it.
- Sun Mar 17, 2019 11:00 am
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Reducing Power
- Replies: 3
- Views: 599
Reducing Power
Why is it that the lower the standard reduction potential, the higher the reducing power, and the higher the standard reduction potential, the stronger the oxidizing agent? I believe that is the trend, if I'm not mistaken and I have that committed to memory but I don't understand why that is the cas...
- Sun Mar 17, 2019 10:57 am
- Forum: Arrhenius Equation, Activation Energies, Catalysts
- Topic: Final
- Replies: 5
- Views: 899
Re: Final
There will probably be a higher emphasis on stuff after both the Midterm and Test 2, (kinetics especially) as those are topics we have yet to be tested on at all.
- Sun Mar 17, 2019 10:54 am
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Inert Electrodes
- Replies: 3
- Views: 587
Re: Inert Electrodes
Thank you so much for your prompt reply, appreciate it!
- Sun Mar 17, 2019 10:51 am
- Forum: Administrative Questions and Class Announcements
- Topic: Saying Thank You to Dr. Lavelle
- Replies: 490
- Views: 579621
Re: Saying Thank You to Dr. Lavelle
Dr. Lavelle, I've been a freshman student in both your 14A and 14B class this year and I just wanted to say thank you so much for going above and beyond with the amount of effort you've poured into all of us making sure that we always have more resources than we could ever ask for and always seeming...
- Sun Mar 17, 2019 10:47 am
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Inert Electrodes
- Replies: 3
- Views: 587
Inert Electrodes
Can we always use Platinum as an inert electrode for our cell diagrams when one is needed or are there others that must be used in certain cases?
- Sun Mar 17, 2019 10:45 am
- Forum: General Science Questions
- Topic: Easy ways to remember
- Replies: 5
- Views: 845
Re: Easy ways to remember
Also I don't have a mnemonic for it but be sure to remember the degeneracy equation (W=X^n) as I don't believe that one is given to us on our formula sheet.
- Sun Mar 17, 2019 10:39 am
- Forum: Second Order Reactions
- Topic: Half Life for Second Order Reactions
- Replies: 3
- Views: 857
Half Life for Second Order Reactions
Why is it that for First Order Reactions the time required for the reaction to go to one fourth remaining is double the half life, but this is not true for second order reactions, as in problem 7B13 from the 7th edition?
- Sun Mar 17, 2019 10:37 am
- Forum: Work, Gibbs Free Energy, Cell (Redox) Potentials
- Topic: Wmax
- Replies: 3
- Views: 876
Re: Wmax
Wmax is directly proportional to the Gibbs free energy so it will be at its highest when Delta G is at its highest as well.
- Sun Mar 17, 2019 1:48 am
- Forum: Balancing Redox Reactions
- Topic: Reducing power
- Replies: 1
- Views: 484
Re: Reducing power
Using standard reduction potentials. I believe a lower standard reduction potential indicates a higher reducing power and thus a higher standard reduction potential indicates a higher oxidizing power.
- Sat Mar 16, 2019 7:42 pm
- Forum: Entropy Changes Due to Changes in Volume and Temperature
- Topic: Question 9.19
- Replies: 1
- Views: 490
Re: Question 9.19
I believe you are heating the liquid water to its boiling point, vaporizing the liquid water at its boiling point into a gas , then cooling the gaseous to the desired temperature, while adding together the entropy for each of these separate stages. Though I can't be sure as I can't seem to find the ...
- Sat Mar 16, 2019 7:37 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Inert Conductor
- Replies: 3
- Views: 376
Re: Inert Conductor
I believe in lecture he said that Mercury will only be used as an inert conductor if it present in the reaction initially but I"m not entirely sure, sorry. But I believe Platinum should be used if an inert conductor is needed unless Mercury is part of the reaction.
- Sat Mar 16, 2019 7:35 pm
- Forum: Arrhenius Equation, Activation Energies, Catalysts
- Topic: Solubility Product
- Replies: 1
- Views: 264
Re: Solubility Product
I don't believe the 5N section is part of our syllabus?
- Sat Mar 16, 2019 7:34 pm
- Forum: General Rate Laws
- Topic: Rate Constant
- Replies: 1
- Views: 445
Re: Rate Constant
The integrated rate laws for zero order, first order, and second order reactions all include the rate constant and time. The differential form of these rate laws expresses teh rate of reaction. And by substituting the final concentration for half the initial in the integrated rate laws, we can solve...
- Sat Mar 16, 2019 7:30 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: using molarity vs moles
- Replies: 2
- Views: 498
Re: using molarity vs moles
Could you be more specific? What kind of a problem are you talking about?
- Sat Mar 16, 2019 7:26 pm
- Forum: Reaction Mechanisms, Reaction Profiles
- Topic: Catalyst in rate law?
- Replies: 6
- Views: 8198
Re: Catalyst in rate law?
Well they aren't present in the final equation but are in the initial step so I suppose they could be in the rate law for one specific step but not the overall rate law? But I'm not sure honestly, sorry.
- Sat Mar 16, 2019 7:24 pm
- Forum: Arrhenius Equation, Activation Energies, Catalysts
- Topic: Determining catalysts
- Replies: 2
- Views: 274
Re: Determining catalysts
I believe you would use the definition of an intermediate and a catalyst to determine that. By definition, an intermediate is not present at the start or end of a reaction, it is created and used up within the process of the reaction. In this case none of the components fit that definition. On the c...
- Sat Mar 16, 2019 7:09 pm
- Forum: Van't Hoff Equation
- Topic: neutral pH not 7?
- Replies: 6
- Views: 1050
Re: neutral pH not 7?
pH 7 is neutral at STP but not for all temperatures as Kw is temperature dependent.
- Sat Mar 16, 2019 6:52 pm
- Forum: Reaction Mechanisms, Reaction Profiles
- Topic: HW problem
- Replies: 2
- Views: 886
Re: HW problem
It is not an intermediate as it is present at the beginning of the reaction. Intermediates are not present at the start or end of a reaction and are created and used up within the process of the reaction. HBrO is present at the beginning, thus making it a potential catalyst I believe.
- Sat Mar 16, 2019 6:50 pm
- Forum: Work, Gibbs Free Energy, Cell (Redox) Potentials
- Topic: Reduction Potentials
- Replies: 2
- Views: 479
Re: Reduction Potentials
Yes I believe that's true; you reverse a reaction in order to have the highest resulting cell potential when you add the two together.
- Sat Mar 16, 2019 6:41 pm
- Forum: Reaction Mechanisms, Reaction Profiles
- Topic: Intermediates in Rate Law
- Replies: 4
- Views: 464
Re: Intermediates in Rate Law
No if something is an intermediate it is created and used up within the reaction steps and therefore I do not believe it should be present in a rate law.
- Sat Mar 16, 2019 6:39 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Reducing/oxidizing power?
- Replies: 2
- Views: 319
Re: Reducing/oxidizing power?
I believe if something has a low reduction potential it has strong reducing power and a high reduction potential signifies strong oxidizing power.
- Sat Mar 16, 2019 6:37 pm
- Forum: Balancing Redox Reactions
- Topic: Emf
- Replies: 1
- Views: 408
Re: Emf
It's Ecell sometimes written as E0cell
- Sat Mar 16, 2019 6:30 pm
- Forum: General Science Questions
- Topic: Easy ways to remember
- Replies: 5
- Views: 845
Re: Easy ways to remember
LEO says GER for Redox reactions:
Losing Electrons = Oxidation
Gaining Electrons = Reduction
Losing Electrons = Oxidation
Gaining Electrons = Reduction
- Sat Mar 16, 2019 5:59 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: 6M7 7th edition
- Replies: 2
- Views: 279
Re: 6M7 7th edition
You don't need to use an equation just compare standard reduction potentials.
- Sat Mar 16, 2019 5:41 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: 6L7 b 7th edition
- Replies: 1
- Views: 231
Re: 6L7 b 7th edition
There are two reactants in this equation, hydogen cation and hydroxide anion. Analyzing both their reduction potentials, we see that hydrogen cation has the stronger standard reduction potential, thus it must be reduced and the hydroxide reaction must be reversed and it must be oxidized. By balancin...
- Sat Mar 16, 2019 5:31 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: 6L7 c 7th edition
- Replies: 1
- Views: 258
Re: 6L7 c 7th edition
Based on lecture, I believe it is because the goal of a cell diagram is to provide directions for how to conduct the described cell and to build that solid state cell hydroxide must be present in the form of a solid compound as the solid state cell could not be built using just aqueous hydroxide, bu...
- Sun Jan 13, 2019 6:21 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Different Concentrations [ENDORSED]
- Replies: 1
- Views: 155
Re: Different Concentrations [ENDORSED]
You can convert between partial pressure and molar concentrations using the Ideal Gas Law:
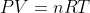
RT)
RT)

- Sun Jan 13, 2019 6:17 pm
- Forum: Ideal Gases
- Topic: Definition of an Ideal Gas [ENDORSED]
- Replies: 2
- Views: 271
Re: Definition of an Ideal Gas [ENDORSED]
An ideal gas is one which perfectly obeys the Ideal Gas Law: PV = nRT.
All collisions between molecules are elastic, kinetic energy and momentum are conserved, and the law is obeyed exactly, without any discrepancies.
All collisions between molecules are elastic, kinetic energy and momentum are conserved, and the law is obeyed exactly, without any discrepancies.
- Sun Jan 13, 2019 6:15 pm
- Forum: Ideal Gases
- Topic: Pressure and Temperature [ENDORSED]
- Replies: 2
- Views: 447
Re: Pressure and Temperature [ENDORSED]
Temperature is directly proportional to the average kinetic energy of molecules of a gas. By increasing the pressure these molecules are placed in a more compact setting, causing them to bounce off each other more frequently, and thus move faster. As these molecules move faster, the temperature incr...
- Sun Dec 09, 2018 1:46 pm
- Forum: Electronegativity
- Topic: Trend of Electronegativity
- Replies: 18
- Views: 4250
Re: Trend of Electronegativity
Electronegativity increases as you go up the periodic table as the energy level is lower so electrons are held closer to the nucleus with lower atomic radii and an increased effective nuclear charge. As you go across the periodic table electronegativity also increases as the energy level remains the...
- Sun Dec 09, 2018 10:59 am
- Forum: Biological Examples
- Topic: Cisplatin
- Replies: 2
- Views: 476
Re: Cisplatin
In cisplatin the molecule is organized so that both binding sites are on the same side of the molecule so it can bind twice to the same strand of DNA stopping cell division.
- Sun Dec 09, 2018 10:20 am
- Forum: Calculating the pH of Salt Solutions
- Topic: konstant?
- Replies: 5
- Views: 549
Re: konstant?
Do you mean the equilibrium constant? That represents the ratio of products over reactants in a reaction at equilibrium.
- Sun Dec 09, 2018 10:18 am
- Forum: Identifying Acidic & Basic Salts
- Topic: Which salts produce which solutions
- Replies: 1
- Views: 395
Re: Which salts produce which solutions
Salts that contain the conjugate base of a weak acid will raise pH by removing protons from water to generate hydroxide. Sodium acetate is a good example of this.
- Sun Dec 09, 2018 10:14 am
- Forum: Polarisability of Anions, The Polarizing Power of Cations
- Topic: Polarizability vs. Polarizing Power
- Replies: 2
- Views: 511
Re: Polarizability vs. Polarizing Power
Perfect, thank you so much!
- Sun Dec 09, 2018 1:05 am
- Forum: Determining Molecular Shape (VSEPR)
- Topic: 2E 7 7th edition
- Replies: 1
- Views: 327
Re: 2E 7 7th edition
The answer book is correct. The formula should be AX3E as there are three atoms bonded to the central atom, one oxygen atom and two chlorine atoms.
- Sun Dec 09, 2018 12:49 am
- Forum: Significant Figures
- Topic: Quantum World, Test 2: SigFigs
- Replies: 1
- Views: 430
Re: Quantum World, Test 2: SigFigs
1 sig fig
- Sun Dec 09, 2018 12:47 am
- Forum: Bronsted Acids & Bases
- Topic: Bronsted acid
- Replies: 9
- Views: 1041
Re: Bronsted acid
Because it donates a proton to become a stable anion (Br-)
- Sun Dec 09, 2018 12:46 am
- Forum: Acidity & Basicity Constants and The Conjugate Seesaw
- Topic: Strong/weak acids and bases
- Replies: 2
- Views: 333
Re: Strong/weak acids and bases
I think he just wanted us to memorize them but generally speaking many strong acids are either polyprotic or the conjugate acid of a halogen anion (with HF being a notable exception).
- Sun Dec 09, 2018 12:45 am
- Forum: Ionic & Covalent Bonds
- Topic: Ionic vs.covalent bonding
- Replies: 4
- Views: 767
Re: Ionic vs.covalent bonding
Ionic bonding usually involves a metal cation and a non metal anion and a transference of charge.
Covalent bonding usually involves two nonmetal atoms and a sharing of electrons in a bond, (no charge only potential dipoles)
Covalent bonding usually involves two nonmetal atoms and a sharing of electrons in a bond, (no charge only potential dipoles)
- Sun Dec 09, 2018 12:43 am
- Forum: Air Pollution & Acid Rain
- Topic: Final
- Replies: 8
- Views: 1171
Re: Final
Pages 490-491 in the 7th Edition have a small blurb on this.
- Sun Dec 09, 2018 12:42 am
- Forum: Air Pollution & Acid Rain
- Topic: Final
- Replies: 8
- Views: 1171
Re: Final
I don't think you need to know anything specifically about acid rain just the concepts involved in that example from the book regarding the reaction of carbonic acid (or a similar strong acid) and water.
- Sun Dec 09, 2018 12:16 am
- Forum: Bohr Frequency Condition, H-Atom , Atomic Spectroscopy
- Topic: Practice problem
- Replies: 2
- Views: 554
Re: Practice problem
The question focuses on using this quantum equation for hydrogen: E_{n}=\frac{hR_{h}}{n^2} Manipulating the equation with the information given, (the energy released as the electron went from one energy level to another) you get: E=\frac{hR_{h}}{n_{f}^2}-\frac{hR_{h}}{n_{i}^2} Where: E = The energy ...
- Sat Dec 08, 2018 11:54 pm
- Forum: Hybridization
- Topic: Hybrid Orbitals
- Replies: 1
- Views: 421
Re: Hybrid Orbitals
It seems to be asking for they hybridization of the second carbon in the chain. That carbon is attached to two other carbons, the first in the chain with a single bond and the third with a triple bond. As such it has two regions of electron density and thus exhibits a hybridization of sp.
- Sat Dec 08, 2018 11:51 pm
- Forum: Amphoteric Compounds
- Topic: Amphoteric vs. Amphiprotic
- Replies: 1
- Views: 374
Re: Amphoteric vs. Amphiprotic
An amphoteric substance can act as either an acid or a base. An amphiprotic substance is a more specific type of amphoteric substance that can easily accept or donate hydrogen ions.
- Sat Dec 08, 2018 11:43 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Lone pair locations
- Replies: 1
- Views: 229
Re: Lone pair locations
I would assume their orientation as in bond angles and shape and VSEPR formulas. How they would experience repulsion and orient themselves far apart from each other such as in a square planar shape.
- Sat Dec 08, 2018 11:41 pm
- Forum: Air Pollution & Acid Rain
- Topic: practice problems?
- Replies: 1
- Views: 475
Re: practice problems?
There is an informational blurb with some example equations in section 6.E.4 on pages 490-491 of the seventh edition.
- Sat Dec 08, 2018 11:38 pm
- Forum: Conjugate Acids & Bases
- Topic: Can someone check if I'm right
- Replies: 2
- Views: 624
Re: Can someone check if I'm right
Keep in mind the hydronium ion has a 1+ charge as H30+ but yes that is correct. Also note that both HSO4- and H20 are amphoteric. But yes you are correct. :)
- Sat Dec 08, 2018 11:24 pm
- Forum: Polarisability of Anions, The Polarizing Power of Cations
- Topic: Polarizability vs. Polarizing Power
- Replies: 2
- Views: 511
Polarizability vs. Polarizing Power
What is the difference between polarizability and polarizing power?
- Sat Dec 08, 2018 11:05 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Seesaw and t shape angles
- Replies: 2
- Views: 752
Re: Seesaw and t shape angles
Less than 120, also in the case of T-Shape versus seesaw both sets of bonds you've referred to are less than 90 degrees but indicating which is specifically lower is not usually important as long as you state that they are each lower than 90.
- Sat Dec 08, 2018 10:47 pm
- Forum: Calculating the pH of Salt Solutions
- Topic: Salt Solution pH — 7th Edition 6.D.11
- Replies: 2
- Views: 405
Re: Salt Solution pH — 7th Edition 6.D.11
You're right it would dissociate into the K+ and F- ions; however, F- is the conjugate base of hydofluoric acid, HF, which is a WEAK acid not a strong acid, and therefore does still affect pH.
- Sat Dec 08, 2018 10:38 pm
- Forum: *Molecular Orbital Theory (Bond Order, Diamagnetism, Paramagnetism)
- Topic: Pi bonds
- Replies: 1
- Views: 421
Re: Pi bonds
I'm not sure but I believe that this is outside the scope of our course as it is from section 2.G.1 in the textbook and the entire 2.G section is absent from the syllabus.
- Sat Dec 08, 2018 10:16 pm
- Forum: Lewis Acids & Bases
- Topic: Lewis Acid
- Replies: 6
- Views: 648
Re: Lewis Acid
Bromine is in Group 4 of the Periodic Table while Fluorine is in Group 2. Based on periodic trends and atomic mass, Bromine is a heavier atom with a much larger atomic radius. As such, the single bond in HBr is much longer than the single bond in HF. Longer bonds are weaker so hydrobromic acid has a...
- Mon Oct 15, 2018 10:06 pm
- Forum: Photoelectric Effect
- Topic: Photoelectric Effect Module
- Replies: 3
- Views: 1523
Photoelectric Effect Module
I had a question about one of the parts of the Photoelectric Effect Module Post Assessment. Question 30 specifically. The given information is as follows: Light hits a sodium metal surface and the velocity of the ejected electron is 6.61 x 10^5 m/s. The work function for sodium is 150.6 kJ/mol. The ...
- Mon Oct 15, 2018 9:28 pm
- Forum: *Particle in a Box
- Topic: Hw assignment?
- Replies: 7
- Views: 1299
Re: Hw assignment?
I believe we are still on the quantum section for homework since we haven't covered anything further so we aren't prepared for homework on sections that have never been discussed in lecture. Also my TA allowed us to do homework from the high school chem review section for both Week 1 and Week 2 sinc...
- Mon Oct 15, 2018 9:25 pm
- Forum: Heisenberg Indeterminacy (Uncertainty) Equation
- Topic: What the equation is used for
- Replies: 5
- Views: 512
Re: What the equation is used for
I'm confused as well and am not entirely sure but I think the equation is more conceptually used to prove that at this scale the means of measurement affects the outcome like Dr. Lavelle emphasized. I think it just proves that by measuring either momentum or distance of an electron in this type of a...
- Fri Oct 05, 2018 5:17 am
- Forum: Accuracy, Precision, Mole, Other Definitions
- Topic: diatomic elements
- Replies: 12
- Views: 9945
Re: diatomic elements
One cheap mnemonic device to remember these is: Never Have Fear Of Ice CoLd Bread
Never: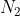 Nitrogen
Nitrogen
Have: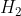 Hydrogen
Hydrogen
Fear: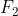 Fluorine
Fluorine
Of: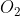 Oxygen
Oxygen
Ice: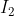 Iodine
Iodine
CoLd: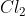 Chlorine
Chlorine
Bread: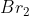 Bromine
Bromine
Never:
Have:
Fear:
Of:
Ice:
CoLd:
Bread:
- Fri Oct 05, 2018 3:32 am
- Forum: Molarity, Solutions, Dilutions
- Topic: (Typo??) HW L.35 - 7th edition
- Replies: 3
- Views: 432
Re: (Typo??) HW L.35 - 7th edition
I have the seventh edition book and solutions manual and I had the exact same issue as you. My third equation in the book is also listed as FeBr_2+Na_2CO_3->NaBr+CO_2+Fe_3O_4 But since the solutions manual uses Fe_3Br_8 I'm assuming that's a typo like you said and just using that as the first reacta...
- Fri Oct 05, 2018 1:55 am
- Forum: Significant Figures
- Topic: Significant Figures for E.21 (b)
- Replies: 4
- Views: 462
Significant Figures for E.21 (b)
Question E.21 (b) asks you to calculate the moles and number of molecules in 25.92 mg of hydrogen fluoride. I tried to solve the question but ended up getting an answer with the incorrect number of significant figures and I can't explain why: \frac{25.92 mg HF}{1}*\frac{1gHF}{1000mgHF}*\frac{1molHF}...

