Search found 81 matches
- Wed Mar 18, 2020 12:58 am
- Forum: Administrative Questions and Class Announcements
- Topic: Chem 14BL
- Replies: 1
- Views: 532
Chem 14BL
Repost because I accidentally put this on the chem 14A section . Hey all! I know this is a little off topic, but is anybody else registered for 14BL next quarter? I know they basically said the show goes on, but I genuinely have no idea how we are going to do a lab completely remotely. Does anyone ...
- Wed Mar 18, 2020 12:37 am
- Forum: Administrative Questions and Class Announcements
- Topic: Chem Final Typo
- Replies: 8
- Views: 829
Re: Chem Final Typo
ayushibanerjee06 wrote:I put none of these just to be safe, maybe it wasn't a typo.
I know he said it wasn't a typo, but I really hope that people who thought it may have been get credit for that question because it was pretty convincing lol.
- Tue Mar 17, 2020 12:24 pm
- Forum: Balancing Redox Reactions
- Topic: Pt in Cell Diagram
- Replies: 14
- Views: 937
Re: Pt in Cell Diagram
Venus_Hagan 2L wrote:if the reactants and products of the half-reaction are not in solid form, you need some type of solid metal as the electrode. Usually, you use Pt(s) because it is a fairly non-reactive metal.
Would any other non-reactive metals be used as a substitute? Or will it always be platinum?
- Tue Mar 17, 2020 11:45 am
- Forum: Administrative Questions and Class Announcements
- Topic: final grades
- Replies: 12
- Views: 1080
Re: final grades
ayushibanerjee06 wrote:Eugene Chung 3F wrote:Does this class have a curve at the end?
there usually is, but I don't know if there will be one this time since the final was online.
The only curve policy I know is the C- for anyone with a 50% or higher. I'm not sure if there is any curve for the entire class as far as I know.
- Mon Mar 16, 2020 4:33 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Chem Community Posts Due Date
- Replies: 13
- Views: 1139
Re: Chem Community Posts Due Date
We have until wednesday now!!!
- Mon Mar 16, 2020 3:02 pm
- Forum: Student Social/Study Group
- Topic: Number of Chemistry Community Posts
- Replies: 45
- Views: 2564
Re: Number of Chemistry Community Posts
Did anyone else take chem 14A last year. I took it Fall 18 and we only needed 30 chemistry community posts back then. Does that mean I only need to post a total of 80 times?
- Mon Mar 16, 2020 2:17 pm
- Forum: Administrative Questions and Class Announcements
- Topic: ENDGAME Review Session
- Replies: 71
- Views: 5667
Re: ENDGAME Review Session
Thanks for all your help Lyndon. Your review packets have been such a big help through this series. I wish you the best of luck in all your future endeavors.
- Mon Mar 16, 2020 1:02 pm
- Forum: Student Social/Study Group
- Topic: Post All Chemistry Jokes Here
- Replies: 9651
- Views: 3588555
Re: Post All Chemistry Jokes Here
Have you heard about that one chemist who was reading a book about helium?
He just couldn't put it down ;)
He just couldn't put it down ;)
- Mon Mar 16, 2020 12:29 pm
- Forum: Administrative Questions and Class Announcements
- Topic: final grades
- Replies: 12
- Views: 1080
Re: final grades
In addition to being able to see our grades, does anyone also know if we will be able to review our answers? I would like to see the questions I got wrong. How will we be able to see these? Is he releasing the final exams online? We'll be able to see specifically what we missed because we took the ...
- Mon Mar 16, 2020 12:15 pm
- Forum: Administrative Questions and Class Announcements
- Topic: final grades
- Replies: 12
- Views: 1080
Re: final grades
In addition to being able to see our grades, does anyone also know if we will be able to review our answers? I would like to see the questions I got wrong.
- Sun Mar 15, 2020 7:55 am
- Forum: Student Social/Study Group
- Topic: Final Jitters
- Replies: 457
- Views: 357221
Re: Final Jitters
To be honest the only thing keeping me sane is the 50% policy to pass this course. With chem being my weakest subject, that's the only reason I'm able to pass this course.
- Sun Mar 15, 2020 7:52 am
- Forum: Administrative Questions and Class Announcements
- Topic: Chem Community Posts Due Date
- Replies: 13
- Views: 1139
Re: Chem Community Posts Due Date
Close to last chem community post!
- Sun Mar 15, 2020 7:51 am
- Forum: Administrative Questions and Class Announcements
- Topic: Saying Thank You to Dr. Lavelle
- Replies: 490
- Views: 546941
Re: Saying Thank You to Dr. Lavelle
Dr Lavelle, Chem is by far my weakest subject, and I put off taking this course until I could take it with you. Thank you for all the available resources you provided for us in order to succeed in this course. Without it, I would not have been able to pass. Best wishes to all of your future courses!...
- Sun Mar 15, 2020 7:48 am
- Forum: Phase Changes & Related Calculations
- Topic: phase change
- Replies: 6
- Views: 536
Re: phase change
Can the enthalpy increase AND the temperature remains the same while there still not being a phase change? Like I'm imagining the enthalpy of water at a positive value but without becoming a gas? Is that possible? I was wondering the same thing. Would any change in either of these, no matter to wha...
- Sat Mar 14, 2020 12:24 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Half/Rxn & Balanced Equations for galvanic cells.
- Replies: 8
- Views: 569
Re: Half/Rxn & Balanced Equations for galvanic cells.
Mandeep Garcha 2H wrote:Pt(s) is added to either the anode or cathode (or even both) when there is no solid metal conductor present.
Is pt the only metal that we use? at least for the purpose of what we're doing in chem 14B?
- Sat Mar 14, 2020 12:22 pm
- Forum: Phase Changes & Related Calculations
- Topic: phase change
- Replies: 6
- Views: 536
Re: phase change
Like the diagram in class, you can tell that there is a phase change when the heat or enthalpy is increasing, but the temperature is not increasing, or delta T is zero. Can the enthalpy increase AND the temperature remains the same while there still not being a phase change? Like I'm imagining the ...
- Sat Mar 14, 2020 12:19 pm
- Forum: Thermodynamic Definitions (isochoric/isometric, isothermal, isobaric)
- Topic: External force
- Replies: 6
- Views: 452
Re: External force
If I understand correctly, external forces are present in both reversible and irreversible processes due to work.
- Sat Mar 14, 2020 12:16 pm
- Forum: Thermodynamic Definitions (isochoric/isometric, isothermal, isobaric)
- Topic: Isochoric/isometric
- Replies: 4
- Views: 521
Re: Isochoric/isometric
Both are interchangeable, just use what you prefer. They both refer to a constant volume within the process.
- Sat Mar 14, 2020 12:13 pm
- Forum: Thermodynamic Definitions (isochoric/isometric, isothermal, isobaric)
- Topic: text problem 4A.5
- Replies: 3
- Views: 315
Re: text problem 4A.5
If not explicitly given, just remember how any change in temperature and pressure in the final/initial values means that the equation is irreversible.
- Sat Mar 14, 2020 12:12 pm
- Forum: Thermodynamic Definitions (isochoric/isometric, isothermal, isobaric)
- Topic: test problem 4A.5 continued
- Replies: 3
- Views: 390
Re: test problem 4A.5 continued
I'm not exactly sure of the derivations for both, but I would just correlate each equation to irreversible and reversible respectively. Just know which of those equations to use given its reversible or not.
- Sat Mar 14, 2020 11:20 am
- Forum: Administrative Questions and Class Announcements
- Topic: Chemistry community posts
- Replies: 3
- Views: 316
Re: Chemistry community posts
I took Chem 14A last year, do you think I will be fine if I have 70 total? Back when I took the class we only needed 30 posts total, and now it's 50.
- Sat Mar 14, 2020 11:10 am
- Forum: Zero Order Reactions
- Topic: All reactants zero order?
- Replies: 7
- Views: 643
Re: All reactants zero order?
In theory I do believe this is possible, however I am not of what an example would be. Could somebody please give a real life application in which this would be the case.
- Sat Mar 14, 2020 11:08 am
- Forum: Arrhenius Equation, Activation Energies, Catalysts
- Topic: Catalysts
- Replies: 8
- Views: 516
Re: Catalysts
Homogeneous are in the same phase and move around the designated volume like it was a reactant or product. When it is a heterogeneous, it is a different phase and the reactants rest on the surface and are adsorbed. What would this difference mean for the reaction? Like how would a homogeneous/heter...
- Sat Mar 14, 2020 11:03 am
- Forum: Reaction Mechanisms, Reaction Profiles
- Topic: Reaction Profile
- Replies: 5
- Views: 415
Reaction Profile
For outline 6, chemical kinetics, one of the things we should be able to do is interpret/draw a reaction profile. Can somebody please give a step by step on how exactly reaction profiles are made, where they come from, and what they show us?
- Sat Mar 14, 2020 10:21 am
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: Enthalpy
- Replies: 26
- Views: 1207
Re: Enthalpy
Fiona Latifi 1A wrote:Examples of state functions include density, internal energy, enthalpy, and entropy.
Could you also give examples of what would not be a state function?
- Sat Mar 14, 2020 9:31 am
- Forum: Calculating Work of Expansion
- Topic: Choosing work equation
- Replies: 10
- Views: 811
Re: Choosing work equation
For an irreversible expansion, use w=-P(deltaX). For a reversible expansion (isothermal), use w=-nRT(ln deltaV2/deltaV1). Would the reversibility/irreversibility of a system be given explicitly in a problem, or would that be something that we have to figure out for ourselves? If so, how would we de...
- Mon Mar 09, 2020 8:32 pm
- Forum: Reaction Mechanisms, Reaction Profiles
- Topic: Pre-Equilibrium Approach
- Replies: 2
- Views: 216
Pre-Equilibrium Approach
With regards to discussing the pre-eq approach today in class, how to we determine the fast and slow bimolecular dimerization reactions? Would that be given or is it something that is logically inferred?
- Thu Mar 05, 2020 1:35 am
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Half/Rxn & Balanced Equations for galvanic cells.
- Replies: 8
- Views: 569
Half/Rxn & Balanced Equations for galvanic cells.
In homework problem 6L3, Platinum appears on both the cathode and anode side of the diagram. I was wondering what this means exactly, and what needs to be done depending on which side it's on?
- Wed Mar 04, 2020 6:09 pm
- Forum: Balancing Redox Reactions
- Topic: Homework Problem 6K.1
- Replies: 3
- Views: 289
Re: Homework Problem 6K.1
the balanced reduction reaction is missing a 6e- on the left side in order to balance the charges. so the oxidation reaction is multiplied by 3 so that the 2e- can balance with the 6e- from the reduction So for the final balanced redox reaction, we just have to make sure that the electrons cancel o...
- Wed Mar 04, 2020 4:26 pm
- Forum: Balancing Redox Reactions
- Topic: Homework Problem 6K.1
- Replies: 3
- Views: 289
Homework Problem 6K.1
For problem 6K.1 in the homework, the last step is creating a completely balanced redox reaction with the balanced half reactions. Can somebody please explain to me why the oxidation reaction is multiplied by 3 in order to produce the final balanced redox rxn. The balanced oxidization reaction is: {...
- Mon Mar 02, 2020 5:36 pm
- Forum: Balancing Redox Reactions
- Topic: Basic vs Acidic Conditions.
- Replies: 6
- Views: 476
Basic vs Acidic Conditions.
With regards to balancing redox reactions, would it be appropriate to make the assumption that under acidic conditions, H2O would always be on the product (right) side of the equation, and that under basic conditions, H2O would always be on the reactant (left) side?
- Mon Mar 02, 2020 10:34 am
- Forum: Administrative Questions and Class Announcements
- Topic: Study guide
- Replies: 7
- Views: 609
Re: Study guide
I found the midterm and final study guide posted by Lydon Bui to be very helpful. He did the same for chem 14a. Other than that I don't know of any study guides that are given out by the instructor and ta's.
- Sun Feb 16, 2020 10:28 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: 4D.23
- Replies: 2
- Views: 254
Re: 4D.23
We subtract twice the value because it is asking for the total reaction enthalpy and there are 2 mol of NO. If was asking for the enthalpy for the formation of 1 mol, then the initial value would be your answer.
- Sun Feb 16, 2020 10:19 pm
- Forum: Calculating Work of Expansion
- Topic: Choosing work equation
- Replies: 10
- Views: 811
Re: Choosing work equation
For an irreversible expansion, use w=-P(deltaX). For a reversible expansion (isothermal), use w=-nRT(ln deltaV2/deltaV1). Would the reversibility/irreversibility of a system be given explicitly in a problem, or would that be something that we have to figure out for ourselves? If so, how would we de...
- Sun Feb 16, 2020 10:14 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: Enthalpy and Heat
- Replies: 3
- Views: 308
Re: Enthalpy and Heat
Matthew Chan 1B wrote:Heat is the form of energy transfer from a one temperature to another, like from hot to cold. Enthalpy is the heat transfer at a constant pressure. So the heat added or lost by the system is the enthalpy change.
Would constant pressure be the only necessary criteria for calculating enthalpy?
- Sun Feb 16, 2020 10:06 pm
- Forum: Entropy Changes Due to Changes in Volume and Temperature
- Topic: S = 0
- Replies: 21
- Views: 1184
Re: S = 0
S just means entropy, rather than change in entropy that should be denoted as ΔS. Entropy is almost never 0 (the entropies of all perfect crystals approach zero as the absolute temperature approaches zero; "perfect crystal" refers to a substance in which all the atoms are in a perfectly o...
- Sun Feb 16, 2020 10:00 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Why does only Temp affect K?
- Replies: 10
- Views: 17057
Re: Why does only Temp affect K?
Temperature affects K c because the forward reaction is either exothermic or endothermic. If the forward reaction is endothermic, then the K c value will be larger when the temperature is higher since the forward reaction will absorb the added heat and produce a greater proportion of products to re...
- Wed Feb 12, 2020 3:11 am
- Forum: Administrative Questions and Class Announcements
- Topic: Pizza Rolls REVIEW Session DOWNLOAD HERE
- Replies: 67
- Views: 5731
Re: Pizza Rolls REVIEW Session DOWNLOAD HERE
Can someone help me solve problem 3B? I wasnt able to make it to the review session and the enthalpy of fusion is causing me problems Hi! someone answered a similar question above but for 3B you are given more info than you think. First you need to realize that the ice cream both warms in solid pha...
- Mon Feb 10, 2020 9:55 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Molar Concentration Direction.
- Replies: 2
- Views: 174
Molar Concentration Direction.
In the example Dr. Lavelle did in class today regarding the 0.482 mol N2 and 0.933 mol O2, why do we assume that O2 changes its molar concentration by -X? What part of the givens indicate how molar concentration would change?
- Sun Jan 26, 2020 11:36 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: 4A Thinking Point
- Replies: 2
- Views: 116
Re: 4A Thinking Point
Would there be any other analogies/examples of this. I understand what the comment above me is saying, but I don't think my idea of being "reversible" in this instance is correct.
- Sun Jan 26, 2020 11:33 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Heat Capacity of Water
- Replies: 6
- Views: 897
Re: Heat Capacity of Water
Just because the values would be different, does not mean the rate will be. The rate is constant and can be applied to both Celsius and Kelvin since they are "proportional"
- Sun Jan 26, 2020 11:28 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Different Enthalpy Strategies
- Replies: 5
- Views: 182
Re: Different Enthalpy Strategies
Depending on what's given in the question, you would decide how to calculate your bond enthalpy. Some methods may not be usable if you don't have enough info given, or just information which would allow you to solve in a particular way.
- Sun Jan 26, 2020 11:23 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Standard State
- Replies: 2
- Views: 95
Re: Standard State
The term standard state refers to the state of an element at 25 degrees celsius and 1 atm. The majority are solids with few exceptions. I wouldn't say that this is something we calculate, rather memorize those which are gasses and liquids since there are only 13.
- Sun Jan 26, 2020 11:17 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Predicting Molar Heat Capacity
- Replies: 3
- Views: 225
Re: Predicting Molar Heat Capacity
I believe Professor Lavelle said something about correlating with size. As the person above me said, complexity (higher mass) would probably mean it has a higher heat capacity in comparison to a simple compound with smaller mass.
- Sun Jan 26, 2020 11:12 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Pure solids & liquids
- Replies: 5
- Views: 345
Re: Pure solids & liquids
I was actually wondering the same thing. I understand that it means they are to be excluded from equilibrium calculations. However, are there any practical uses of this information? Like does knowing the state give us any other hints other than to exclude it.
- Sun Jan 12, 2020 9:48 pm
- Forum: Non-Equilibrium Conditions & The Reaction Quotient
- Topic: Partial Pressure
- Replies: 3
- Views: 153
Re: Partial Pressure
I'm pretty sure you need to know either pressure or temperature to solve for one another. Given a compound, I don't think you can do much with that alone so the answer to your question would be no.
- Sun Jan 12, 2020 9:34 pm
- Forum: Non-Equilibrium Conditions & The Reaction Quotient
- Topic: Calculating for Pressure
- Replies: 5
- Views: 205
Re: Calculating for Pressure
Temperature is needed in order to use the ideal gas law (solving for pressure in your case). You can't solve for two unknowns, so short answer is no, not possible.
- Fri Jan 10, 2020 10:29 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Why does only Temp affect K?
- Replies: 10
- Views: 17057
Re: Why does only Temp affect K?
Kc is dependent on temperature. A change in T would change the value of Kc, and this value will scale with respect to what kind of reaction is occurring (endothermic/exothermic).
- Fri Jan 10, 2020 10:20 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Comparing K and Q
- Replies: 6
- Views: 282
Re: Comparing K and Q
We must calculate Q in order to tell us where in the reaction we are. We compare the Q value to K and we can determine which way the reaction is favored, or if they are equal, then reaction is at equilibrium.
- Fri Jan 10, 2020 10:03 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: 5I.5
- Replies: 4
- Views: 168
Re: 5I.5
You did everything right, change 1.8 to 1.18 and you will get the correct outputs.
- Sat Dec 08, 2018 11:47 pm
- Forum: Administrative Questions and Class Announcements
- Topic: FINAL PRACTICE - Lyndon's Churro Review Session [ENDORSED]
- Replies: 118
- Views: 21303
Re: FINAL PRACTICE - Lyndon's Churro Review Session [ENDORSED]
For 39, why can't PH3 have hydrogen bonds?
- Sat Dec 08, 2018 6:47 pm
- Forum: Dipole Moments
- Topic: Strengths if Intermolecular Forces
- Replies: 1
- Views: 471
Strengths if Intermolecular Forces
I remember earlier in the year when we went over the different types of bonding that take place within molecules (dispersion, dipole-dipole, hydrogen). Can somebody explain how to identify these bonds by looking at the lewis structure? Also I remember that there was an order which went from weakest ...
- Sat Dec 08, 2018 6:37 pm
- Forum: Lewis Acids & Bases
- Topic: 6th edition 12.17
- Replies: 5
- Views: 504
Re: 6th edition 12.17
Alma Carrera 3C wrote:Is there a way to tell based on the lewis structures or do we just have to remember this?
I would just remember the trends. (Nonmetal oxides = acidic, metal oxides = basic) BaO is basic because when it reacts with water, Ba(OH)2 is formed which means OH is formed.
- Fri Dec 07, 2018 11:52 pm
- Forum: Bronsted Acids & Bases
- Topic: Question 12.9 (Sixth Edition)
- Replies: 1
- Views: 356
Re: Question 12.9 (Sixth Edition)
I'm actually pretty stumped on this one as well, I'm not exactly sure what the text means by writing the net ionic equations.
- Fri Dec 07, 2018 8:51 pm
- Forum: Resonance Structures
- Topic: Delocalization
- Replies: 6
- Views: 1193
Re: Delocalization
In resonance, a lone pair can sometimes be in different locations with respect to the configuration. For example, drawing the lewis structure for 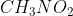 would result in resonance between two different structures in which the lone electron pair can be placed on either oxygen atom.
would result in resonance between two different structures in which the lone electron pair can be placed on either oxygen atom.
- Fri Dec 07, 2018 8:41 pm
- Forum: Formal Charge and Oxidation Numbers
- Topic: Formal Charge vs Partial charge
- Replies: 4
- Views: 2286
Re: Formal Charge vs Partial charge
Formal charge is used primarily in lewis structures and molecular formulas and the charges (if any) on both must be equivalent. This gives us an indication of the stability of a molecule, with FC=0 being optimal. Partial charge shows us electronegativity, and is a basis for determining how badly a m...
- Fri Dec 07, 2018 8:22 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Lone pairs on octahedral electron density
- Replies: 3
- Views: 393
Re: Lone pairs on octahedral electron density
With every other VSEPR shape, 1 removed molecule removed will affect the configuration in different ways with respect to the one removed, thus resulting in different possible bond angles in some. With octahedral, no matter which one is removed, the result will always be the same.
- Fri Dec 07, 2018 8:18 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Determining polar and non polar molecules from a lewis structure
- Replies: 8
- Views: 2889
Re: Determining polar and non polar molecules from a lewis structure
How do you know HCN is polar or non polar? There is a negative charge on the N and a positive charge on the H. When drawing the lewis structure, you notice that the CN bond is a triple bond with a lone pair while the HC bond is only a single bond. Thus, the dipole moments do not cancel which makes ...
- Fri Dec 07, 2018 8:11 pm
- Forum: Amphoteric Compounds
- Topic: What makes something amphoteric?
- Replies: 6
- Views: 821
Re: What makes something amphoteric?
Compounds that demonstrate both acidic and basic properties, and can act as either an acid or a base are charactarized as amphoteric. Metal oxides as well as hydroxides are examples of amphoteric compound, with its acidic or basic nature being in respect to their oxidizations.
- Fri Dec 07, 2018 8:04 pm
- Forum: Calculating pH or pOH for Strong & Weak Acids & Bases
- Topic: Ranking strong acids and bases
- Replies: 4
- Views: 759
Re: Ranking strong acids and bases
Polarity and electronegativity are the easiest way to determine strength. Strength of acidity is based upon its ability to lose a proton. Strong acids dissociate completely in water while weak acids only dissolve partially.
- Tue Dec 04, 2018 2:24 pm
- Forum: Sigma & Pi Bonds
- Topic: Strength of Sigma and Pi Bonds
- Replies: 5
- Views: 1118
Re: Strength of Sigma and Pi Bonds
The overlap of pi bonds are smaller than the overlap of sigma bonds, making pi bonds easier to break apart. Hence, sigma bonds are stronger.
- Tue Dec 04, 2018 2:20 pm
- Forum: Sigma & Pi Bonds
- Topic: Pi bonds
- Replies: 4
- Views: 668
Re: Pi bonds
Think of a line intersecting a figure 8 in 3D. If you combine the two then the sides of the 8's would touch "88". Rotating one of them away from you or towards you would break the bond between, which is why pi bonds do not permit the rotation about an axis.
- Tue Dec 04, 2018 2:12 pm
- Forum: Hybridization
- Topic: P orbital
- Replies: 7
- Views: 650
Re: P orbital
Unless there is only one region of electron density, then there will always be a "p" proceeding the "s".
- Tue Dec 04, 2018 2:08 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: polarity
- Replies: 11
- Views: 844
Re: polarity
With that logic, it would make sense that due to the presence of a lone pair then the shape would not be symmetrical, therefore electrons would not be evenly shared. However I would say drawing out the structure just to demonstrate and prove its polarity. I'm not sure that your reasoning would suffi...
- Tue Dec 04, 2018 1:59 pm
- Forum: Amphoteric Compounds
- Topic: amphoteric substances
- Replies: 3
- Views: 415
Re: amphoteric substances
I believe that there is some sort of pattern on the periodic table which begins with Be and goes diagonal downwards ending in Bi. This would be in the case of Oxides.
- Sun Nov 11, 2018 11:43 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Molecular Shape
- Replies: 5
- Views: 601
Re: Molecular Shape
If there are no lone pairs on the central atom, then the configuration is linear which means the bond angle will always be 180 degrees.
- Sun Nov 11, 2018 11:38 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Naming bond angles
- Replies: 1
- Views: 252
Re: Naming bond angles
I would say that the names of the molecular shapes should be memorized. They correspond with line pairs of electrons which can be seen through lewis structures. Linear, Trigonal Planar, Tetrahedral, Trigonal Bipyramidal, and Octahedral, are all possible configurations corresponding to VSEPR theory a...
- Sun Nov 11, 2018 11:27 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Bond angles
- Replies: 3
- Views: 349
Re: Bond angles
I believe that it has something to do with the shape of the molecule. VSEPR configurations include shapes such as: linear, trigonal, tetrahedral, etc... and since we know that each of the electrons distance themselves equally then we can use this to determine the angle. Ex: 2 molecules would be 180 ...
- Sun Nov 11, 2018 11:22 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Polar and Non-polar
- Replies: 3
- Views: 360
Re: Polar and Non-polar
Polarity relies on the length of the bonds between molecules. I would say that its safe to assume that if the electronegativity is obviously within or beyond the parameters of polarity then we can jump to a conclusion wether or not it is. Other than a brief glance at this pattern, there wouldn't be ...
- Sun Nov 04, 2018 8:51 pm
- Forum: Formal Charge and Oxidation Numbers
- Topic: Formal Charge on Central Atom
- Replies: 9
- Views: 1578
Re: Formal Charge on Central Atom
Formal charge is utilized in order to predict reactivity with the closer the charge being to 0, the more stable it becomes.
- Sun Nov 04, 2018 8:33 pm
- Forum: Resonance Structures
- Topic: Purpose of Resonance Hybrids
- Replies: 4
- Views: 440
Re: Purpose of Resonance Hybrids
To my understanding, the easiest way for me to understand resonance hybridization is that it gives us the set of all possibilities/configurations for a particular molecule and that the actual representation would be a combination of all those possibilities.
- Sun Nov 04, 2018 8:22 pm
- Forum: Octet Exceptions
- Topic: Valence Electron
- Replies: 2
- Views: 951
Re: Valence Electron
The big concept behind valence electrons are that they are ones in the orbitals furthest out and unfilled. 5s^2 and 5p^3 are not filled orbitals and therefore the number of valence electrons for the element Sb = 5. For Mn, the electron configuration would be [Ar]3d^5 4s^2. In this case as Venya said...
- Sun Oct 28, 2018 11:52 pm
- Forum: Octet Exceptions
- Topic: do we nee to memorize the octet exceptions?
- Replies: 6
- Views: 1017
Re: do we nee to memorize the octet exceptions?
Kailie_Giebink_1E wrote:will we need to know this for the test??
I would memorize these just in case. Overall it's couldn't hurt just to memorize the 4 exceptions, but it would benefit more if you knew the reasoning and concepts behind. They can help you understand electron configurations as a concept all together.
- Sun Oct 28, 2018 11:39 pm
- Forum: Lewis Structures
- Topic: Lines that Represent Bonds
- Replies: 7
- Views: 621
Re: Lines that Represent Bonds
As far as I understand, both methods represent the same configuration but the solid lines are used because it makes things more obvious in terms of shared/bonded electrons.
- Sun Oct 28, 2018 11:35 pm
- Forum: Ionic & Covalent Bonds
- Topic: Roman numerals next to element
- Replies: 8
- Views: 6143
Re: Roman numerals next to element
The roman numeral proceeding the element name represents the charge of the element.
- Fri Oct 12, 2018 6:04 pm
- Forum: DeBroglie Equation
- Topic: "Wave-like Properties" of Matter
- Replies: 6
- Views: 274
Re: "Wave-like Properties" of Matter
From what Dr. Lavelle told us in lecture, it is to my understanding that with such small margins, he would not make us determine what would be acceptable to apply wave length properties to. The general concept was that wave length properties are only applied to matters at the micro level as opposed ...
- Fri Oct 12, 2018 6:00 pm
- Forum: Photoelectric Effect
- Topic: Variable for frequency
- Replies: 6
- Views: 570
Re: Variable for frequency
The variables representing different values can be manipulated to your benefit, if you were to chose any other variable that you would know for a fact you would be able to distinguish, I don't see a reason why you shouldn't. The answer won't be any different as long as the true representations of th...
- Fri Oct 12, 2018 5:57 pm
- Forum: Bohr Frequency Condition, H-Atom , Atomic Spectroscopy
- Topic: Solving for frequency
- Replies: 3
- Views: 393
Re: Solving for frequency
Hertz (Hz) is the unit of measurement used when denoting frequency which is why it would be proper to express a frequency in terms of hertz.
- Fri Oct 05, 2018 1:13 pm
- Forum: Empirical & Molecular Formulas
- Topic: Compound Names
- Replies: 9
- Views: 835
Re: Compound Names
Nitric acid is a compound that may be used more as the quarter goes on, it is one of the simpler ones but for many of us we wouldn't be able to solve the problem due to the composition not being given. Nitric acid is HNO3 and I think that it would be wise to memorize this for it is not an uncommon c...
- Fri Oct 05, 2018 1:09 pm
- Forum: Empirical & Molecular Formulas
- Topic: Question F9
- Replies: 5
- Views: 493
Re: Question F9
For the question, I believe that all it asked for was to give an answer in the form of a ratio, the way information is given to us I don't think that giving the empirical formula would be necessary. It would not be hard to give both answers though.
- Fri Oct 05, 2018 1:02 pm
- Forum: Balancing Chemical Reactions
- Topic: How To....
- Replies: 16
- Views: 2633
Re: How To....
Knowing the state of a particular element or compound may be essential in balancing chemical equations, however it may be very difficult to know many of them by heart. In my opinion, knowing the state of the matters are helpful in visualizing a particular reaction but even if not given, the problem ...

