Search found 66 matches
- Thu Mar 14, 2019 9:07 pm
- Forum: *Enzyme Kinetics
- Topic: Do we need to know enzyme kinetics for the final? [ENDORSED]
- Replies: 4
- Views: 908
Do we need to know enzyme kinetics for the final? [ENDORSED]
Do we need to know enzyme kinetics for the final?
- Thu Mar 14, 2019 9:06 pm
- Forum: Arrhenius Equation, Activation Energies, Catalysts
- Topic: 15.67 6th Edition (Catalyst)
- Replies: 1
- Views: 216
15.67 6th Edition (Catalyst)
Why did they use Ea,cat=(75/125) instead of Ea,cat=(125-75) to help find the factor by which the rate of the reaction changed?
- Wed Mar 13, 2019 11:18 pm
- Forum: Arrhenius Equation, Activation Energies, Catalysts
- Topic: 15.61 (6th edition)
- Replies: 1
- Views: 257
15.61 (6th edition)
Can someone explain where they got the equation they use to solve for Ea:
ln(k'/k)=(Ea/R)[(1/T)-(1/T')]
ln(k'/k)=(Ea/R)[(1/T)-(1/T')]
- Sat Mar 09, 2019 2:37 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Today's Review Session (Sat 12-2)
- Replies: 1
- Views: 328
Today's Review Session (Sat 12-2)
Hi, did anyone go to today's review session (Mar. 9, 12-2pm) and would be willing to post the notes/practice? I really wanted to go but woke up very sick. If you could help a girl out you'd be the MVP of the day!
- Thu Mar 07, 2019 10:03 pm
- Forum: Kinetics vs. Thermodynamics Controlling a Reaction
- Topic: Lecture Notes Week 9 Wednesday (3/6)
- Replies: 3
- Views: 474
Re: Lecture Notes Week 9 Wednesday (3/6)
Here you go :)
- Thu Mar 07, 2019 9:54 pm
- Forum: Second Order Reactions
- Topic: 7th Edition 7A.11 2nd Order RXN
- Replies: 2
- Views: 391
Re: 7th Edition 7A.11 2nd Order RXN
I think so. If you expanded it to be k[A][A] then you would be able to find that A could be two different reactants.
- Thu Mar 07, 2019 9:51 pm
- Forum: Kinetics vs. Thermodynamics Controlling a Reaction
- Topic: What does unique rate of reaction mean?
- Replies: 6
- Views: 618
Re: What does unique rate of reaction mean?
The unique raate of the reaction can be solved for using this formula: -(1/a)(d[a]/dt) Conceptually it is average reaction rate adjusted for the stoichiometric coefficient so that the consumptions of each product and reactant are equal. "Unique reaction rate is the average reaction rate divided...
- Sun Mar 03, 2019 1:40 pm
- Forum: First Order Reactions
- Topic: Dividing Experiments to Find Order
- Replies: 4
- Views: 455
Re: Dividing Experiments to Find Order
Another question that goes off of this is, can you have an order be a fraction or do they have to be whole numbers?
- Sun Mar 03, 2019 12:33 pm
- Forum: Kinetics vs. Thermodynamics Controlling a Reaction
- Topic: Purpose of Kinetics
- Replies: 6
- Views: 1721
Purpose of Kinetics
Okay, I know this is a pretty basic question but, I think it is the source of all my confusion. What is kinetics used for and why do we care? I get that it has to do with reaction rates but are we just finding how different variables affect the reaction?
- Sun Mar 03, 2019 12:26 pm
- Forum: Work, Gibbs Free Energy, Cell (Redox) Potentials
- Topic: Why does -Wsys = Wmax?
- Replies: 1
- Views: 221
Why does -Wsys = Wmax?
Why does -Wsys= Wmax in reference to Gibbs free energy?
- Sun Feb 24, 2019 9:19 pm
- Forum: Balancing Redox Reactions
- Topic: Problem 14.1, 6th ed.
- Replies: 3
- Views: 391
Re: Problem 14.1, 6th ed.
Could it be that you have to balance oxygen with water so you then have to balance the newly added hydrogens for acidic solutions? And then in the basic solution, you balance the hydrogen by adding water and then have to balance the newly added oxygens?
Just an idea
Just an idea
- Sun Feb 24, 2019 9:12 pm
- Forum: Work, Gibbs Free Energy, Cell (Redox) Potentials
- Topic: Spontaneous Process
- Replies: 2
- Views: 274
Re: Spontaneous Process
Delta G is negative for a spontaneous reaction.
- Sun Feb 24, 2019 2:18 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Gibbs energy
- Replies: 2
- Views: 308
Re: Gibbs energy
Delta G^o is the standard free energy change based on standard state formation of 1 mol of the substance. Delta G is the free energy change of the substance not in the standard state.
- Sun Feb 17, 2019 6:34 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Gibbs energy
- Replies: 1
- Views: 211
Re: Gibbs energy
He used 0 for Gibbs Free energy to find the critical point at which the liquid and gas phase both exist. At this temperature deltaG is zero so, a temperature higher than the critical temp found will be spontaneous because deltaG is negative. A temperature lower will mean a positive deltaG so the rev...
- Tue Feb 12, 2019 7:32 pm
- Forum: Phase Changes & Related Calculations
- Topic: rotational v vibrational energy
- Replies: 5
- Views: 886
Re: rotational v vibrational energy
Rotational would be around an axis, think of it making a circle. Vibrational is just back and forth on one axis, think of walking back and forth on a line.
- Tue Feb 12, 2019 12:03 pm
- Forum: Administrative Questions and Class Announcements
- Topic: DOWNLOAD SESSION WORKSHEETS HERE - Sun 7-9PM (Karen)
- Replies: 179
- Views: 23850
Re: DOWNLOAD SESSION WORKSHEETS HERE - Sun 7-9PM (Karen)
For #6, if heat is negative and therefore losses heat why is the reaction endothermic?
Nevermind I don't know why I put a negative there. I know heat is positive now.
Nevermind I don't know why I put a negative there. I know heat is positive now.
- Tue Feb 12, 2019 11:58 am
- Forum: Administrative Questions and Class Announcements
- Topic: DOWNLOAD SESSION WORKSHEETS HERE - Sun 7-9PM (Karen)
- Replies: 179
- Views: 23850
Re: DOWNLOAD SESSION WORKSHEETS HERE - Sun 7-9PM (Karen)
For #6, how do we know that work is equal to 0? I was wondering the same thing. What I came up with was that is you use pv=nrt to find deltaV then you can see that since pressure, temperature, and R are constant the change in volume will depend on the change in moles. The equation has a 1:1 ratio f...
- Mon Feb 04, 2019 8:26 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Bond enthalpy vs Bond Enregy
- Replies: 3
- Views: 300
Bond enthalpy vs Bond Enregy
Are bond enthalpy and bond energy the same thing? If not what is the difference?
- Mon Feb 04, 2019 7:53 pm
- Forum: Third Law of Thermodynamics (For a Unique Ground State (W=1): S -> 0 as T -> 0) and Calculations Using Boltzmann Equation for Entropy
- Topic: Small Error in S vs. Large Error in W
- Replies: 1
- Views: 236
Re: Small Error in S vs. Large Error in W
This just means that a large difference in W will only result in a small change in S.
- Mon Feb 04, 2019 7:44 pm
- Forum: Entropy Changes Due to Changes in Volume and Temperature
- Topic: Residual Entropy
- Replies: 3
- Views: 348
Re: Residual Entropy
Residual entropy is the difference between entropy not at equilibrium and the state that the molecule is closest to zero (temperature). It is just a way to measure entropy which is ultimately a way to measure work.
- Sat Feb 02, 2019 3:38 pm
- Forum: Phase Changes & Related Calculations
- Topic: Chem equations vs. Physics equation for Phase change
- Replies: 1
- Views: 294
Chem equations vs. Physics equation for Phase change
I'm currently in physics 5B and for phase changes, we used similar but different equations for phase changes and temperature changes. I was just curious how they relate to each other (other than the fact that they are for the same concept). Physics Equations: - For phase change: mL (m is mass and id...
- Sat Feb 02, 2019 3:21 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Absorbing and Releasing Heat
- Replies: 2
- Views: 2065
Re: Absorbing and Releasing Heat
If you think about something that is very hot, like a cup of coffee, and you put it on a room temperature table does it stay hot when you come back to get it after an hour or two? Objects want to reach equilibrium and that includes thermal equilibrium. As the coffee sits out the heat (which is the t...
- Sat Feb 02, 2019 3:12 pm
- Forum: Thermodynamic Systems (Open, Closed, Isolated)
- Topic: Reversible reaction
- Replies: 3
- Views: 404
Re: Reversible reaction
In a reversible reaction, the reactants can for the products and the products can also produce the reactants.
In an IRreversible reaction, once the products are formed through reactions they cannot form the reactants because they are reacting to form other products.
In an IRreversible reaction, once the products are formed through reactions they cannot form the reactants because they are reacting to form other products.
- Sat Jan 26, 2019 4:23 pm
- Forum: Phase Changes & Related Calculations
- Topic: Sublimation
- Replies: 6
- Views: 564
Re: Sublimation
In response to the question about dry ice, it is special in the way that carbon in the form of dry ice goes directly to a gas phase and doesn't become a liquid. Sublimation occurs because the phase change happens at temperatures and pressures that can't allow the compound to be liquid. It requires a...
- Sat Jan 26, 2019 4:11 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Hess's Law
- Replies: 5
- Views: 461
Re: Hess's Law
Pretty much. You can add (or subtract) equations together to get the desired reaction that you need the enthalpy for. Once you have decided what needs to be added or subtracted you use their enthalpies, making sure the signs match if that equation is endo- or exothermic.
- Wed Jan 23, 2019 10:44 pm
- Forum: Ideal Gases
- Topic: Ice Table with quadratic equation on bottom
- Replies: 3
- Views: 335
Ice Table with quadratic equation on bottom
Is it possible to have an end result of the ice table that gives you only an x in the numerator and the quadratic equation is on the bottom? I have been looking at a problem over and over to make sure I wasn't making a mistake. If it is possible how do you use algebra to solve for x. If it isn't wha...
- Thu Jan 17, 2019 9:31 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: writing equations
- Replies: 1
- Views: 157
Re: writing equations
If you are finding the pH or pOH, the best place to start is to figure out if it will produce H3O or OH. Once you know that you can figure out if you are missing an element that would have to come from water. If it is an acid you will most likely always have to add water to write out the equation, f...
- Thu Jan 17, 2019 9:20 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Relationship between pH and pKa
- Replies: 2
- Views: 356
Relationship between pH and pKa
What is the full relationship between pH and pKa?
- Thu Jan 17, 2019 12:02 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Test Topics for Week 3 [ENDORSED]
- Replies: 1
- Views: 282
Re: Test Topics for Week 3 [ENDORSED]
The best place to start is to go through the homework for outlines 1 and 2; anything you needed to do the homework with is what you'll need to know. The big ones being: Kc= ([C]^c*[D]^d)/([A]^a*[B]^b) Kp= ((PC)^c*(PD)^d)/((PA)^a*(PB)^b) P meaning partial pressures pV=nRT Q=[P]/[R] for reactions not ...
- Wed Jan 16, 2019 7:54 pm
- Forum: Non-Equilibrium Conditions & The Reaction Quotient
- Topic: Homework 6th Edition #61
- Replies: 1
- Views: 148
Re: Homework 6th Edition #61
What chapter is the question you have in?
- Sun Jan 13, 2019 11:21 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Using variables in ICE tables
- Replies: 2
- Views: 252
Using variables in ICE tables
Can someone explain when you should use variables such as X in an ICE table versus finding the change based on stoichiometric coefficients before being plugged into the table? In one of the Audio-Visual Learning Modules (I think equilibrium part 2) Dr.Lavelle went ahead and used mole ratios to find ...
- Sun Jan 13, 2019 11:14 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: 11.7 6th Edition
- Replies: 1
- Views: 134
Re: 11.7 6th Edition
First, you have to physically count the individual molecules that are single and the molecules that are still bonded to find the moles of reactants and the moles of products. Once you have those values you multiply them by 0.1 because that is the partial pressure given to you in the problem and you ...
- Thu Jan 10, 2019 10:12 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Q and K
- Replies: 4
- Views: 292
Re: Q and K
Can you clarify please because if you calculate Q the same way you calculate K how can they be different values?
- Wed Dec 05, 2018 8:33 pm
- Forum: Dipole Moments
- Topic: Dipole Moment of C and H
- Replies: 3
- Views: 720
Dipole Moment of C and H
Even though there is a difference of electronegativity between C and H, why do we ignore it and say there is no dipole moment? Is it because it isn't significant enough?
- Wed Dec 05, 2018 8:31 pm
- Forum: Hybridization
- Topic: Composition
- Replies: 1
- Views: 196
Composition
In a review session, the LA said that if they ask for the composition of the bond then you determine it for the sigma bond and pi bonds separately. The textbook shows them together. What is right/ how do you determine the coefficient for composition?
- Wed Dec 05, 2018 8:23 pm
- Forum: Conjugate Acids & Bases
- Topic: Neutralization Reactions
- Replies: 6
- Views: 979
Neutralization Reactions
How do you write a neutralization reaction if they give you the product? How do you decide what acid/base to use?
- Sun Dec 02, 2018 10:57 am
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: why do we care about coordination
- Replies: 2
- Views: 168
Re: why do we care about coordination
They are a way to examine compounds with transition metals. They have biological significance, think about the chemotherapy example Prof. Lavelle used in class.
- Sun Dec 02, 2018 10:51 am
- Forum: Naming
- Topic: naming coordination compounds for the final
- Replies: 3
- Views: 409
Re: naming coordination compounds for the final
Be able to fully name compounds is the best way to make sure you are ready for the final. There are good example questions in the book to help you.
- Sun Dec 02, 2018 10:46 am
- Forum: Bronsted Acids & Bases
- Topic: Bronsted and Lewis Acids/Bases
- Replies: 1
- Views: 393
Re: Bronsted and Lewis Acids/Bases
From what I understand they do. An acid will gain an electron pair and donate a proton. An oxidizing agent gains electrons, so I think you can say an acid is an oxidizing agent. A base is a proton acceptor and donates electron pairs. A reducing agent loses electrons, so I think you can say a base is...
- Sun Dec 02, 2018 10:27 am
- Forum: Bronsted Acids & Bases
- Topic: Are Bronsted and Lewis acids the same thing?
- Replies: 2
- Views: 605
Re: Are Bronsted and Lewis acids the same thing?
Yes, they are the same thing. The difference is just what characteristic of the molecule you are using to define it as an acid or base. A Lewis acid is a molecule that accepts electrons, a Bronsted acid is a molecule that donates a proton. An acid will have both of these characteristics. H2SO4 is an...
- Wed Nov 21, 2018 4:25 pm
- Forum: Hybridization
- Topic: bonds
- Replies: 4
- Views: 183
Re: bonds
As I understood it the sigma bond doesn't become a pi bond, it is that a sigma bond is there and a pi bond is added next to it. Is this right?
- Wed Nov 21, 2018 4:16 pm
- Forum: Sigma & Pi Bonds
- Topic: Clarification of Pi Bonds and Rotation
- Replies: 3
- Views: 1151
Re: Clarification of Pi Bonds and Rotation
Pi bonds have electron density on either side of the internuclear axis. This means that because of electron repulsion and the regions of electron density formed by the already present sigma bond, the electrons are "locked" into a region where they can remain. There is no room for the elect...
- Wed Nov 21, 2018 4:03 pm
- Forum: Hybridization
- Topic: Purpose of hybridization
- Replies: 4
- Views: 396
Re: Purpose of hybridization
Hybridization is important for bonding. You "mix" the orbitals together to visualize and determine how any valance electrons area available for bonding.
- Fri Nov 16, 2018 8:38 pm
- Forum: Dipole Moments
- Topic: Dipole Fluctuality
- Replies: 1
- Views: 228
Re: Dipole Fluctuality
When the regions of electrons fluctuate in a molecule (call it molecule A) this changes where the dipole interaction with another molecule (call it molecule B) is "located". Since electrons aren't really stationary they cause the region of partial charge to shift back and forth a bit. When...
- Fri Nov 16, 2018 8:30 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: analysis of non-polar molecules without vectors
- Replies: 2
- Views: 271
Re: analysis of non-polar molecules without vectors
When there are no partial charges on any of the atoms, then the molecule will be non-polar (hence no dipole moments). The molecule can still be non-polar if the negative charges on an atom are canceled out by a positive charge on another atom (when the dipole moments are canceled out). To cancel out...
- Fri Nov 16, 2018 8:21 pm
- Forum: Hybridization
- Topic: Sigma and Pi
- Replies: 5
- Views: 445
Re: Sigma and Pi
The reason you can't have two sigma bonds is because of the orientation of the regions of electron concentration. There is always electron repulsion and since a pi bond can't have a rotation, the atoms/ molecules are locked in a specific position. Once a pi bond is used, the following bonds have to ...
- Sun Nov 11, 2018 5:04 pm
- Forum: Bond Lengths & Energies
- Topic: 3.97 6th ed
- Replies: 1
- Views: 266
Re: 3.97 6th ed
Yes, the molecule obeys the octet rule, however, phosphorous can have an expanded octet in other instances. Start by connecting all of the P to each other (literally make sure each P has a line to all the rest). Then apply the valence electron rule, where you make sure you have the correct number. Y...
- Sun Nov 11, 2018 4:59 pm
- Forum: Interionic and Intermolecular Forces (Ion-Ion, Ion-Dipole, Dipole-Dipole, Dipole-Induced Dipole, Dispersion/Induced Dipole-Induced Dipole/London Forces, Hydrogen Bonding)
- Topic: Intermolecular Forces
- Replies: 4
- Views: 426
Re: Intermolecular Forces
I also have a follow-up question to this... In class, we talked about how those forces would be interchangeable with dipole-induced dipole interactions. Does this mean they are the same or for sake of simplicity we treat them the same way?
- Sun Nov 11, 2018 4:55 pm
- Forum: Lewis Structures
- Topic: Expanded Octets
- Replies: 9
- Views: 2839
Re: Expanded Octets
Common elements that can have an expanded octet are sulfur, phosphorous, silicon and chlorine. They can have more than an octet because they have d- orbitals that can hold electrons. These d-orbitals have higher energy than 4s- orbitals so those electrons are used for bonding. Here is a helpful link...
- Sun Nov 11, 2018 4:49 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Chemistry Community Posts
- Replies: 3
- Views: 449
Re: Chemistry Community Posts
The post can be a question you need to be answered about the material, a reply/ answer to someone's post, or a helpful resource. I think if it is a question that is a logistic question (like this one), it may not get points but, double check with your TA about that.
- Sat Nov 03, 2018 8:47 pm
- Forum: Lewis Structures
- Topic: Radicals?
- Replies: 4
- Views: 364
Re: Radicals?
I think so. If I understand correctly, everything up to and including Wednesday's lecture (but not Friday's lecture).
- Sat Nov 03, 2018 8:42 pm
- Forum: Empirical & Molecular Formulas
- Topic: Midterm Practice Problem #1
- Replies: 2
- Views: 2843
Re: Midterm Practice Problem #1
General steps: 1)Convert grams of each element into moles of the element 2)Take moles of each element and multiply by the ratio of that element in the molecule (1 C/ 1 CO2) so that you get grams of the individual element 3) Take those grams from step 2 and convert from grams of the element to mols o...
- Sat Nov 03, 2018 8:16 pm
- Forum: Bond Lengths & Energies
- Topic: Coordinate covalent bonds
- Replies: 1
- Views: 210
Re: Coordinate covalent bonds
A coordinate covalent bond is an example he used in class where H2O is bonded and the electrons are being shared between the H and O but, once they break apart the O is left with 6 electrons and the H are each left with one. In short, the one atom donates both electrons in a bond.
- Sat Nov 03, 2018 7:49 pm
- Forum: Octet Exceptions
- Topic: Which atom gets extra lone pair
- Replies: 2
- Views: 255
Re: Which atom gets extra lone pair
Are you referring to Lewis Structures? If so, I think it is whichever one is more electronegative as long as you apply the valence electron and octet rule correctly.
- Sun Oct 28, 2018 6:57 pm
- Forum: Lewis Structures
- Topic: Lewis Structure
- Replies: 2
- Views: 205
Re: Lewis Structure
You want the Lewis Structure to have the least amount of charges on each atom. If there are charges on specific atoms in the molecule then you want it to be a low number. Here is a video I found helpful: https://www.youtube.com/watch?v=r9Hc71xPMPs This person also does some other videos that were a ...
- Sun Oct 28, 2018 6:49 pm
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: 9C.5 7th Ed.
- Replies: 2
- Views: 396
Re: 9C.5 7th Ed.
Ligands are molecules or ions that have one or more pairs of lone electrons. They typically bond to things that have a positive charge. They are classified by how many pairs they have. The Lewis structure will be a more thorough way to check the pairs of electrons because you have to draw it out and...
- Sun Oct 28, 2018 6:37 pm
- Forum: Wave Functions and s-, p-, d-, f- Orbitals
- Topic: Question 2.33 (Sixth Edition)
- Replies: 2
- Views: 972
Re: Question 2.33 (Sixth Edition)
Energy will increase because it is dependent on n (n increases). n increases from 1 to 2. l increases because it is dependent on n as well (l can be 0 to n-1). The radius of an atom is a function of n ( as you increase the " electron shells" the wider the atom) so it also increases. Think ...
- Thu Oct 18, 2018 10:13 pm
- Forum: Wave Functions and s-, p-, d-, f- Orbitals
- Topic: Wave Function v. Orbital
- Replies: 3
- Views: 206
Re: Wave Function v. Orbital
Also, the orbitals are actually math functions that show the probability of finding an electron in that space (shape).
- Thu Oct 18, 2018 9:48 pm
- Forum: DeBroglie Equation
- Topic: 1.59 6th edition
- Replies: 1
- Views: 132
Re: 1.59 6th edition
Once you use the equation: E=hc(lambda)^-1
you will get energy in joules per photon. You then multiply that by 9.8 x 10^20 which is the number of photons.
(You should get 450 J. )
you will get energy in joules per photon. You then multiply that by 9.8 x 10^20 which is the number of photons.
(You should get 450 J. )
- Mon Oct 15, 2018 9:08 pm
- Forum: DeBroglie Equation
- Topic: Velocity using de Broglie equation
- Replies: 2
- Views: 258
Velocity using de Broglie equation
Why do we use 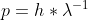
instead of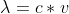 to find the velocity of a neutron?
to find the velocity of a neutron?
instead of
- Sat Oct 13, 2018 10:24 pm
- Forum: Photoelectric Effect
- Topic: Intensity — Photoelectric Effect
- Replies: 1
- Views: 288
Re: Intensity — Photoelectric Effect
There is not a way to calculate intensity/ amplitude using that formula as far as I am aware. If you were looking for a formula to find intensity you may want to use this one: light intensity is proportional to 1/ distance squared (distance being in meters from light source) Also, the relationship b...
- Fri Oct 12, 2018 12:10 pm
- Forum: Photoelectric Effect
- Topic: Equations
- Replies: 3
- Views: 160
Re: Equations
The energy of a photon is determined by the equation hv . This energy is absorbed by the electron that is either ejected (if the energy of the photon is greater than work function) or remains. A key thing to understand that may help answer your question is that the equation (1/2)m e v 2 = hv- work f...
- Fri Oct 12, 2018 11:59 am
- Forum: Properties of Light
- Topic: Oscillation of the Electric and Magnetic Field
- Replies: 1
- Views: 84
Re: Oscillation of the Electric and Magnetic Field
Oscillations of the electric and magnetic field are the wave that light travels in. The oscillation can be measured by using amplitude and wavelength. Amplitude: height up and down of the peak from the middle (see figure 1.7) can be squared to find the intensity of the light (radiation) Wavelength (...
- Tue Oct 02, 2018 3:48 pm
- Forum: Molarity, Solutions, Dilutions
- Topic: Find concentration of ions given more than one solute
- Replies: 2
- Views: 406
Re: Find concentration of ions given more than one solute
Do you think we convert each compound to moles and then just find percent composition?
- Tue Oct 02, 2018 3:44 pm
- Forum: Molarity, Solutions, Dilutions
- Topic: Molarity and Mass
- Replies: 2
- Views: 166
Re: Molarity and Mass
More specifically, take the volume you would like to prepare and multiply that by molarity (moles/liters) which should give you moles. Once you have moles, multiply by the molar mass of the compound (found from adding up the atomic masses). This gives you grams needed to create the solution. If you ...
- Tue Oct 02, 2018 12:32 pm
- Forum: Balancing Chemical Reactions
- Topic: Net Number of Moles
- Replies: 3
- Views: 273
Re: Net Number of Moles
The previous reply is correct. For that question, the net number of moles is 4 because you add up the moles on the left side of the equation (reactants) and then subtract that from the total number of moles on the right side of the equations (products). Follow up question: If there is a net gain the...

