Search found 62 matches
- Tue Mar 12, 2019 1:00 pm
- Forum: Arrhenius Equation, Activation Energies, Catalysts
- Topic: Catalysts Effect on Overall Rxn vs. Step of Rxn
- Replies: 2
- Views: 491
Re: Catalysts Effect on Overall Rxn vs. Step of Rxn
This is also because the the third step of the reaction is not included in the overall rate expression. Thus a catalyst speeding up the third reaction doesn't actually affect the overall rate, as it would only lower the activation energy of the third reaction to make it faster, not the rest of the r...
- Tue Mar 12, 2019 12:53 pm
- Forum: Reaction Mechanisms, Reaction Profiles
- Topic: Frequency Factor
- Replies: 1
- Views: 269
Frequency Factor
Would each reaction have a unique frequency factor? Does the forward and reverse reaction have the same frequency factor?
- Tue Mar 12, 2019 12:49 pm
- Forum: Reaction Mechanisms, Reaction Profiles
- Topic: Rate dependency on [H2O]
- Replies: 1
- Views: 250
Rate dependency on [H2O]
In what circumstances would rate be dependent on the [H2O]?
- Fri Mar 08, 2019 5:41 pm
- Forum: First Order Reactions
- Topic: Order reactions
- Replies: 4
- Views: 532
Re: Order reactions
You can infer from either the units of the rate constant, or the experimental data will tell you.
- Fri Mar 08, 2019 5:38 pm
- Forum: Second Order Reactions
- Topic: pseudo-first-order reaction
- Replies: 4
- Views: 514
Re: pseudo-first-order reaction
For a pseudo first order reaction, the overall reaction could be second order, but because we make one of the reactants be in excess, the concentration of that reactant doesn't really change. Thus we can regard it as a constant, resulting in only the other reactant changing concentration affecting t...
- Fri Mar 08, 2019 5:13 pm
- Forum: Reaction Mechanisms, Reaction Profiles
- Topic: Value of “k” in Kinetics
- Replies: 5
- Views: 806
Re: Value of “k” in Kinetics
The equilibrium constant K is equal to k/k'. Thus if K is greater than 1, it means that k' (the rate of the reverse reaction) is very small or slow. This makes sense since when K is greater than one, the products are favored in the reaction, and vice versa.
- Thu Feb 28, 2019 6:21 am
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Cell Diagram notation
- Replies: 2
- Views: 230
Cell Diagram notation
Why is it that for some notations different species are separated with a comma (ex. Pt| Ti2+, Ti3+||Co2+|Co) while others are separated by a line (ex. Pt|H2|H+||Fe2+,Fe3+|Pt)?
- Thu Feb 28, 2019 6:17 am
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Why can't we add standard cell potentials?
- Replies: 1
- Views: 220
Why can't we add standard cell potentials?
Why do we have to convert cell potentials to  using
using 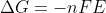 to find unknown cell potentials?
to find unknown cell potentials?
- Thu Feb 28, 2019 6:08 am
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Standard Gibbs free energy of elements
- Replies: 1
- Views: 231
Standard Gibbs free energy of elements
Why is the Gibbs free energy of formation for elements always zero?
- Thu Feb 21, 2019 1:00 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Inert Electrodes
- Replies: 2
- Views: 324
Inert Electrodes
Can someone explain how inert electrodes work and which ones we should use in our cell diagrams?
- Thu Feb 21, 2019 12:47 pm
- Forum: Work, Gibbs Free Energy, Cell (Redox) Potentials
- Topic: Units for Gibbs
- Replies: 8
- Views: 946
Re: Units for Gibbs
I don't think it matters whether the final answer is in J or kJ, but make sure when you're plugging them in the equation they're in the same units.
- Thu Feb 21, 2019 12:45 pm
- Forum: Work, Gibbs Free Energy, Cell (Redox) Potentials
- Topic: Oxidation Potential
- Replies: 2
- Views: 307
Re: Oxidation Potential
Yes, since the tables given are usually of the standard reduction potentials, to get the standard oxidation potential you simply have to flip the sign.
- Fri Feb 15, 2019 8:50 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Extensive v. Intensive Property of Heat Capacities
- Replies: 3
- Views: 458
Re: Extensive v. Intensive Property of Heat Capacities
The value of the heat capacity will change depending on how much of the substance there is, while the specific heat capacity will always remain constant regardless of how much stuff you have.
- Fri Feb 15, 2019 8:48 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: When does delta U equal zero?
- Replies: 17
- Views: 8322
Re: When does delta U equal zero?
If the reaction is isothermal, delta U=0.
- Fri Feb 15, 2019 8:45 pm
- Forum: Student Social/Study Group
- Topic: Post All Chemistry Jokes Here
- Replies: 9651
- Views: 3638253
Re: Post All Chemistry Jokes Here
Q: What is Josaiah Willard Gibbs' favorite holiday?
A: ThanksGibbing
A: ThanksGibbing
- Thu Feb 07, 2019 1:25 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Cv vs Cp
- Replies: 3
- Views: 450
Re: Cv vs Cp
If volume is constant, there can be no work done from expansion, thus the heat provided to the system is converted to work, which explains the lower Cv value.
- Thu Feb 07, 2019 1:10 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Bomb calorimeters vs polystyrene cup calorimeters
- Replies: 3
- Views: 612
Bomb calorimeters vs polystyrene cup calorimeters
During lecture we mentioned bomb calorimeters and normal calorimeters, what is the difference between the two? What conditions and equations apply to each of them?
- Thu Feb 07, 2019 1:10 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Bomb calorimeters vs polystyrene cup calorimeters
- Replies: 2
- Views: 314
Bomb calorimeters vs polystyrene cup calorimeters
During lecture we mentioned bomb calorimeters and normal calorimeters, what is the difference between the two? What conditions and equations apply to each of them?
- Thu Feb 07, 2019 1:07 pm
- Forum: Concepts & Calculations Using Second Law of Thermodynamics
- Topic: Reversible vs irreversible
- Replies: 3
- Views: 558
Re: Reversible vs irreversible
Irreversible processes have changes in pressure and volume that occur so drastically that the system does not have enough time to reach equilibrium, and thus the system and surroundings are not at equilibrium while work is being done. However, since reversible processes occur with an infinite amount...
- Thu Jan 31, 2019 1:10 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Comparing SHC
- Replies: 3
- Views: 487
Re: Comparing SHC
Not necessarily, SPC of a substance depends on properties such as intermolecular forces. SPC is just a measure of how many Joules it takes to raise 1 gram of a substance by 1 degree Celsius.
- Thu Jan 31, 2019 12:56 pm
- Forum: Phase Changes & Related Calculations
- Topic: Steam vs Boiling Water
- Replies: 10
- Views: 10313
Re: Steam vs Boiling Water
Steam causes worse burns as when it contacts your skin, it's transferring both the energy from the phase change, as well as the additional energy it possesses as a vapor.
- Thu Jan 31, 2019 12:43 pm
- Forum: Thermodynamic Systems (Open, Closed, Isolated)
- Topic: Thermal Equilibrium
- Replies: 5
- Views: 419
Re: Thermal Equilibrium
This is similar to chemical equilibrium we covered last unit, where the energy transfer between the molecules don't stop, but rather there is no net energy movement between the system and the surroundings as the rate of energy transfer is the same.
- Wed Jan 23, 2019 4:48 pm
- Forum: Non-Equilibrium Conditions & The Reaction Quotient
- Topic: Strength of acids based on Ka and pKa
- Replies: 4
- Views: 2072
Strength of acids based on Ka and pKa
Hi!
How can we tell how strong an acid is by looking only at their Ka and pKa value? Does this rule work the same for bases? Thanks.
How can we tell how strong an acid is by looking only at their Ka and pKa value? Does this rule work the same for bases? Thanks.
- Wed Jan 23, 2019 4:44 pm
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Salts
- Replies: 4
- Views: 500
Re: Salts
A salt is composed of a cation and an anion. By examining the dissociated cation or anion you can tell how the pH will change. If the cation is able to give off an H+, it will lower the pH, if the anion is able to accept an H+, it will raise the pH.
- Wed Jan 23, 2019 4:39 pm
- Forum: Ideal Gases
- Topic: Ideal Gas Law
- Replies: 7
- Views: 761
Re: Ideal Gas Law
The ideal gas law can first be used to solve for an unknown property for a gas problem, or it can be rearranged to P=(n/V)RT to convert between concentration and partial pressure for gases.
- Fri Jan 18, 2019 4:46 pm
- Forum: Non-Equilibrium Conditions & The Reaction Quotient
- Topic: Catalysts
- Replies: 7
- Views: 728
Re: Catalysts
Catalysts will speed up both the forward and reverse reaction by lowering the activation energy. This means that equilibrium will be reached more quickly.
- Fri Jan 18, 2019 4:30 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Endothermic
- Replies: 2
- Views: 274
Re: Endothermic
Energy is required for bonds to break, and in the decomposition of halogens more energy is used to break the bond than released in the formation of the products. Thus the overall reaction is endothermic.
- Fri Jan 18, 2019 4:28 pm
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Inert Gases
- Replies: 5
- Views: 627
Re: Inert Gases
Since the gas is inert, it won't react with anything else in the mixture and won't affect concentration levels.
- Wed Jan 09, 2019 11:10 pm
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Catalysts
- Replies: 3
- Views: 290
Re: Catalysts
Catalysts only lower the activation energy for the reaction pathway, they do not change the value of K
- Wed Jan 09, 2019 11:06 pm
- Forum: Non-Equilibrium Conditions & The Reaction Quotient
- Topic: Spontaneous reaction
- Replies: 4
- Views: 366
Re: Spontaneous reaction
A spontaneous reaction is one that favors the formation of products since it is more energetically favorable
- Wed Jan 09, 2019 11:00 pm
- Forum: Ideal Gases
- Topic: Memorization
- Replies: 12
- Views: 1276
Re: Memorization
it's on the constants and equations sheet provided on Dr. Lavelle's website!
- Wed Dec 05, 2018 7:08 pm
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: Unidentate
- Replies: 3
- Views: 404
Re: Unidentate
Polydentates have multiple "bites" which allows them to bind to multiple sites on the transition metal cation. Monodentates only have one "bite" so they are only able to bond to one site. You should also consider the structure of the ligand to see if they are able to be properly ...
- Wed Dec 05, 2018 7:05 pm
- Forum: Identifying Acidic & Basic Salts
- Topic: Identifying the difference
- Replies: 3
- Views: 235
Re: Identifying the difference
Another thing to note is that the conjugate bases of strong acids do not affect the pH of the water, for instance Cl- ion doesn't raise the pH despite it being a conjugate base.
- Wed Dec 05, 2018 7:02 pm
- Forum: Properties & Structures of Inorganic & Organic Acids
- Topic: Carboxyl groups and acids
- Replies: 2
- Views: 347
Re: Carboxyl groups and acids
Acids with an -COOH group are organic acids (carboxylic acids). They create hydronium ions by donating the hydrogen at the end of the molecule to the water molecules.
- Sat Dec 01, 2018 10:12 am
- Forum: Bronsted Acids & Bases
- Topic: What are conjugate acids and bases??
- Replies: 2
- Views: 363
Re: What are conjugate acids and bases??
A conjugate base results when the original acid donates one of its protons. For example, H2CO3 is the acid, and HCO3- is its conjugate base.
- Sat Dec 01, 2018 10:07 am
- Forum: Lewis Acids & Bases
- Topic: solution of weak acids with higher pH
- Replies: 4
- Views: 1468
Re: solution of weak acids with higher pH
Since weak acids do not dissociate 100%, they will produce less H3O+. The concentration of H3O+ will be lower, and results in a higher pH value.
- Sat Dec 01, 2018 10:06 am
- Forum: Lewis Acids & Bases
- Topic: Strong Acid
- Replies: 8
- Views: 1175
Re: Strong Acid
They dissociate completely in water to form hydronium ions. Examples would be HCl, HBr, HNO3 etc.
- Mon Nov 26, 2018 12:17 pm
- Forum: Administrative Questions and Class Announcements
- Topic: DOWNLOAD SESSION WORKSHEETS HERE - Sundays 4-6pm (Karen) [ENDORSED]
- Replies: 135
- Views: 39279
Re: DOWNLOAD SESSION WORKSHEETS HERE - Sundays 4-6pm (Karen) [ENDORSED]
These are so helpful, thank you!
- Tue Nov 20, 2018 4:41 pm
- Forum: Hybridization
- Topic: Unhybridized orbitals
- Replies: 10
- Views: 1422
Re: Unhybridized orbitals
I think hybridized orbitals can be thought of as electrons existing in between the energy levels of both s and p orbitals.
- Tue Nov 20, 2018 4:31 pm
- Forum: Hybridization
- Topic: Regions of electron density
- Replies: 6
- Views: 751
Re: Regions of electron density
Regions of electron density around central atom is equal to the number of hybrid orbitals. For instance, if there are 6 regions of electron density, the structure would be six sp3d2 hybridized bonds.
- Tue Nov 20, 2018 4:24 pm
- Forum: Hybridization
- Topic: S and p character effect on bonds
- Replies: 2
- Views: 372
Re: S and p character effect on bonds
Pi-bonds are always present in double and triple bonds, which makes those shorter and stronger than single bonds, which only consists of only a sigma bond, making it the weaker and longer bond.
- Sat Nov 17, 2018 1:46 pm
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: Sigma bond and Pi bonds
- Replies: 2
- Views: 519
Re: Sigma bond and Pi bonds
A single bond consists of a sigma bond, a double bond consists of a sigma and pi bond, while a triple bond consists of a sigma and two pi bonds. Sigma bonds are formed in 3 conditions: 2 s-orbitals overlap, 1 s-orbital and 1 lobe of a p-orbital overlap, and when one lobe of each p-orbital overlaps w...
- Sat Nov 17, 2018 1:41 pm
- Forum: Polarisability of Anions, The Polarizing Power of Cations
- Topic: Polarizability
- Replies: 3
- Views: 459
Re: Polarizability
Polarizability can be thought of as the ability for a molecule's electrons to be distorted/affected by other charges. Atoms with more electrons typically have a higher polarizability since they are less attracted to the positive nucleus due to shielding of electrons.
- Sat Nov 17, 2018 1:38 pm
- Forum: Dipole Moments
- Topic: Polarity and dipole moment
- Replies: 4
- Views: 529
Re: Polarity and dipole moment
Since the dipoles are equal in magnitude but opposite in direction, they will add to zero, meaning there is no overall dipole for the molecule.
- Thu Nov 08, 2018 11:52 am
- Forum: Lewis Structures
- Topic: Expanded octet
- Replies: 4
- Views: 426
Re: Expanded octet
Since expanded octets apply to all elements Period 3 and on, they have access to their d-orbitals to form more bonds.
- Thu Nov 08, 2018 11:43 am
- Forum: Administrative Questions and Class Announcements
- Topic: Type of calculator
- Replies: 8
- Views: 1109
Re: Type of calculator
Any calculator that doesn't have programming capabilities is acceptable i think, you should be fine.
- Thu Nov 08, 2018 11:42 am
- Forum: Bond Lengths & Energies
- Topic: bond lengths and strengths
- Replies: 9
- Views: 943
Re: bond lengths and strengths
There are more electrons involved with double and triple bonds, which pulls the atoms closer together and contributes to bond strength.
- Tue Oct 30, 2018 6:49 pm
- Forum: Formal Charge and Oxidation Numbers
- Topic: Formal Charge of Ions
- Replies: 9
- Views: 992
Re: Formal Charge of Ions
The overall charge of the sulfate ion is 2-, but the central atom sulfur has a formal charge of 0 based on the FC = V - (L +S/2) formula.
- Tue Oct 30, 2018 4:40 pm
- Forum: Resonance Structures
- Topic: Drawing Resonance Structures
- Replies: 1
- Views: 221
Re: Drawing Resonance Structures
I think benzene is a special case where you add the ring in the middle of the diagram to represent the delocalized electrons. For other resonant structures, you use double arrows to show that each of the structures "shift" between each other.
- Tue Oct 30, 2018 4:30 pm
- Forum: Octet Exceptions
- Topic: central atoms with more than 4 bonds
- Replies: 3
- Views: 497
Re: central atoms with more than 4 bonds
Since sulfur is a p-block element, it's able to have an expanded octet, since it has more room in its energy level to form other bonds.
- Tue Oct 23, 2018 4:39 pm
- Forum: Ionic & Covalent Bonds
- Topic: Electron Configuration of Cations
- Replies: 8
- Views: 892
Re: Electron Configuration of Cations
Taking a look at the Roman numerals, the copper cation can either have +1 or +2 charge.
- Tue Oct 23, 2018 4:20 pm
- Forum: Trends in The Periodic Table
- Topic: Ionization Energies
- Replies: 3
- Views: 468
Re: Ionization Energies
The first ionization energy is the energy required to remove one mole of electrons from a neutral atom, while the second ionization energy is the energy required to remove another mole of electrons from the ion at its already positively charged state.
- Tue Oct 23, 2018 4:07 pm
- Forum: Properties of Light
- Topic: Is light in waves or photons?
- Replies: 10
- Views: 972
Re: Is light in waves or photons?
Light is subject to the wave-particle duality! It exhibits both properties of light, which means you can use applicable wave equations to determine frequency and wavelength etc., while also using equations that show that it's a particle, like the work function equation.
- Thu Oct 18, 2018 11:47 am
- Forum: Photoelectric Effect
- Topic: Intensity
- Replies: 2
- Views: 235
Re: Intensity
Higher intensity of light will not affect the amount of energy the ejected electrons will have. No matter how intense the light is, if it still does not have enough energy to surpass the threshold energy (work function), no electrons will be ejected. Frequency does affect the amount of energy of eje...
- Thu Oct 18, 2018 11:44 am
- Forum: *Particle in a Box
- Topic: Work Function
- Replies: 3
- Views: 710
Re: Work Function
Each metal has a different work function, or the minimum energy that the incidental photons required to eject electrons from the surface. The excess energy is then converted to kinetic energy for the electron in EK = hf - work function
- Thu Oct 18, 2018 11:16 am
- Forum: Quantum Numbers and The H-Atom
- Topic: Psi squared meaning
- Replies: 4
- Views: 2958
Re: Psi squared meaning
ψ^2 is a wave function that describes the probability of finding an electron in a certain area, our TA said for the next quiz there wouldn't be too much about it, but you should know the definition.
- Wed Oct 10, 2018 7:48 pm
- Forum: Molarity, Solutions, Dilutions
- Topic: Changing Units
- Replies: 10
- Views: 831
Re: Changing Units
As long as your final answer is in the correct units the problem asks for, and you're able to cancel out the extra ones properly you should be fine!
- Wed Oct 10, 2018 7:42 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Tutoring? [ENDORSED]
- Replies: 121
- Views: 269341
Re: Tutoring? [ENDORSED]
You can also check the websites for more info on their Step-Up program! I heard it's really helpful and thorough with their explanations.
- Wed Oct 10, 2018 7:37 pm
- Forum: Limiting Reactant Calculations
- Topic: Percent Yield [ENDORSED]
- Replies: 13
- Views: 2899
Re: Percent Yield [ENDORSED]
The actual yield is like the experimental yield of the reaction, something you would get if you performed the reaction in real life. The theoretical yield is the value you get when you do your stoichiometry calculations!
- Wed Oct 03, 2018 1:25 pm
- Forum: General Science Questions
- Topic: Systematic vs random error?
- Replies: 2
- Views: 559
Systematic vs random error?
Hello!
I'm still a little confused between the differences for systematic and random error in labs, could someone please clarify using some examples? Thanks!
I'm still a little confused between the differences for systematic and random error in labs, could someone please clarify using some examples? Thanks!
- Tue Oct 02, 2018 4:00 pm
- Forum: Student Social/Study Group
- Topic: Any necessary data booklets?
- Replies: 2
- Views: 345
Any necessary data booklets?
Hello!
Are there any necessary data booklets (ones that include the periodic table, relevant formulas, etc.) that we should have? Thanks!
Are there any necessary data booklets (ones that include the periodic table, relevant formulas, etc.) that we should have? Thanks!
- Tue Oct 02, 2018 3:28 pm
- Forum: Accuracy, Precision, Mole, Other Definitions
- Topic: Would it be acceptable to use the term concentration (C) for molarity (M) of a solution?
- Replies: 3
- Views: 376
Would it be acceptable to use the term concentration (C) for molarity (M) of a solution?
Hello!
I'm a student from Canada and we've always referred to the molarity of a solution as the concentration (C) of a solution. They're still in the same units (mol/L), and have the same formula (C=n/V), I was just wondering if there is a conventional preference? Thank you!
I'm a student from Canada and we've always referred to the molarity of a solution as the concentration (C) of a solution. They're still in the same units (mol/L), and have the same formula (C=n/V), I was just wondering if there is a conventional preference? Thank you!

