Search found 30 matches
- Tue Nov 27, 2018 12:52 am
- Forum: Hybridization
- Topic: 2.45 7th Edition
- Replies: 1
- Views: 282
Re: 2.45 7th Edition
Image-1.jpg First you need to draw the lewis structure by putting the 3 carbon atoms in the middle and adding the rest of the atoms bonded to the carbon atoms. After you have the lewis structure you need to label the bonds (all single bonds are sigma bonds, double bonds are one sigma and one pi bon...
- Tue Nov 27, 2018 12:39 am
- Forum: Hybridization
- Topic: Pi bonds
- Replies: 2
- Views: 417
Re: Pi bonds
You can also think back to the demonstration Dr. Lavelle did with the white board markers. With one white board marker between your fingers, you can twist you fingers around, however, with two white board markers, you can't move your fingers much without dropping the markers.
- Tue Nov 27, 2018 12:36 am
- Forum: Hybridization
- Topic: hybridization
- Replies: 1
- Views: 265
Re: hybridization
The key thing about hybrid orbitals is that they depend on the number of electron densities around an atom. For example, in CH 4 carbon has 4 areas of electron densities and so it is sp 3 hybridized. Note, hybridization depends on electron densities not bonds, so the same atom carbon in CO 2 is sp h...
- Mon Nov 26, 2018 12:31 pm
- Forum: Hybridization
- Topic: How to find hybridization orbitals 4.35
- Replies: 3
- Views: 368
Re: How to find hybridization orbitals 4.35
yes, first draw the lewis structures for the molecules and then count how many areas of electron densities are around the central atom to figure out the hybridization of the central atom.
- Mon Nov 26, 2018 12:25 pm
- Forum: Hybridization
- Topic: Sigma and pi bond
- Replies: 2
- Views: 324
Re: Sigma and pi bond
The number of pi and sigma bonds is for each structure/resonance structure, so if asked how many pi or sigma bonds a molecule has, you should not add up the pi or sigma bonds of all the resonance structures.
- Fri Nov 16, 2018 12:21 am
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Linear shape alternate forms
- Replies: 3
- Views: 361
Re: Linear shape alternate forms
Don't forget that molecules are 3D!
This isn't a correct VSEPR model since lone pairs aren't atoms, but just to give a sense of depth I used the VSEPR model bond notation to show where the lone pairs of electrons could be placed on a linear molecule.
This isn't a correct VSEPR model since lone pairs aren't atoms, but just to give a sense of depth I used the VSEPR model bond notation to show where the lone pairs of electrons could be placed on a linear molecule.
- Fri Nov 16, 2018 12:13 am
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Formula for determining bond angle
- Replies: 6
- Views: 634
Re: Formula for determining bond angle
We can estimate bond angles based on the molecule's VSEPR shape, however, only experimentally testing for bond angle gives us the actual bond angle between atoms in a molecule.
- Fri Nov 16, 2018 12:03 am
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Bond Angle of Sulfite Ion
- Replies: 2
- Views: 361
Re: Bond Angle of Sulfite Ion
Similar to how the lone pair of electrons in water cause the bond angle between the H atoms to be less than 109.5 degrees, the lone pair of electrons causes the O-S-O angles to also be less than 109.5 degrees (since lone pair-bonding repulsion is stronger than bonding-bonding repulsion and thus the ...
- Thu Nov 15, 2018 11:51 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Repulsion Strength
- Replies: 5
- Views: 525
Re: Repulsion Strength
yes, for example, if you look at SH 2 compared to CH 4 , both molecules have the same number of "things" around the central atom, however, due to differences in lone pair-lone pair repulsion and bonding-bonding repulsion, the two molecules have very different shapes and bond angles. Image-...
- Thu Nov 15, 2018 11:39 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Molecular Shapes
- Replies: 3
- Views: 397
Re: Molecular Shapes
Also, if you think about it, because of lone pair-bonding repulsion compared to bonding-bonding repulsion and the total number of "things" around the central atom, the one lone pair of electrons will cause major VSEPR shape differences in the molecule (in the seesaw shape, the lone pair pu...
- Thu Nov 15, 2018 11:30 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Lewis and VSEPR for I3-
- Replies: 3
- Views: 3135
- Thu Nov 08, 2018 5:12 pm
- Forum: Bond Lengths & Energies
- Topic: Bond Lengths
- Replies: 6
- Views: 618
Re: Bond Lengths
I think all you need to do is have a general understanding of bond lengths and what affects them. For example, in lecture Dr. Lavelle talked about how larger atoms have longer bond lengths (due to electron shielding etc.) and single bonds are generally longer than double bonds which are longer than ...
- Thu Nov 08, 2018 4:56 pm
- Forum: Bond Lengths & Energies
- Topic: Affect of direction of a bond relative to other bonds on bond length
- Replies: 1
- Views: 268
Affect of direction of a bond relative to other bonds on bond length
Does the direction of a bond relative to other bonds in a molecule affect how long it is? For example, are the single bonds in a molecule like tetraphosphorus (P4), which has a tetrahedral structure, shorter than a molecule with only one single bond?
- Thu Nov 08, 2018 4:45 pm
- Forum: Dipole Moments
- Topic: Dipoles Canceling
- Replies: 2
- Views: 500
Re: Dipoles Canceling
It depends on the direction of the dipole moments. For example, in benzene all the dipole moments cancel out so there is no dipole-dipole force. However in water the dipole moments do not cancel out and so H2O has a dipole-dipole force.
- Fri Nov 02, 2018 9:29 pm
- Forum: Lewis Structures
- Topic: 6th edition 41.c
- Replies: 2
- Views: 754
6th edition 41.c
Could someone give me step by step instructions on how they wrote the lewis structure for 41.(c) glycine, H2C(NH2)COOH?
- Fri Nov 02, 2018 12:22 am
- Forum: Formal Charge and Oxidation Numbers
- Topic: Lecture 1 Example
- Replies: 2
- Views: 319
Re: Lecture 1 Example
Yes, in a nitrate ion (NO 3 - ), N has 5 valence electrons, O has 18 valence electrons (6 x 3 = 18), and because this is an anion with a -1 charge, Dr. Lavelle added another e - for a total of 24 e - . So the -1 charge was not due to the formal charge equation, but rather it was already given by NO ...
- Fri Nov 02, 2018 12:13 am
- Forum: Resonance Structures
- Topic: Electron movements
- Replies: 2
- Views: 318
Re: Electron movements
If you think back to when we learned about orbitals, electrons in a molecule are shared/found where the orbitals (of each individual element) overlap. These orbitals show the probability of the electron being found there (there is no way to know exactly where the electron is at a given moment).
- Fri Nov 02, 2018 12:08 am
- Forum: Lewis Structures
- Topic: central atoms
- Replies: 8
- Views: 793
Re: central atoms
Are there any exceptions to the rule that the central atom is the one with the lowest ionization energy?
- Wed Oct 24, 2018 1:13 pm
- Forum: Wave Functions and s-, p-, d-, f- Orbitals
- Topic: Spins
- Replies: 4
- Views: 388
Re: Spins
Spin up and spin down is just a way to describe the electron's angular momentum (it does not mean that the electrons actually spin in place). Spin up corresponds to an m s value of 1/2 and spin down corresponds -1/2. The spin values won't be written directly in the electron configuration notation, h...
- Wed Oct 24, 2018 12:51 pm
- Forum: Quantum Numbers and The H-Atom
- Topic: Magnetic Quantum Number
- Replies: 3
- Views: 505
Re: Magnetic Quantum Number
The magnetic quantum number tells you about the orientation of the electron orbitals. For example, if you think back to the lecture where we talked about the p-orbital and how when l = 1, m l can be -1, 0, or 1 which roughly translates to p x , p y , or p z . Note though that when going from the m l...
- Wed Oct 24, 2018 12:19 pm
- Forum: Wave Functions and s-, p-, d-, f- Orbitals
- Topic: Writing an Electron Configuration Format
- Replies: 3
- Views: 397
Re: Writing an Electron Configuration Format
It depends on what you're being asked for. In general, the 1s^2 2s^2 2p^2 should be fine. 1s^2 2s^2 2px^1 2py^1 is more specific than you usually need because it gives you information about the spin of the electrons in the last p orbital relative to one another (electrons being in pairs or not).
- Fri Oct 19, 2018 11:13 am
- Forum: Properties of Light
- Topic: Microwaves vs. Actual Microwaves
- Replies: 1
- Views: 348
Re: Microwaves vs. Actual Microwaves
Fun fact: according to google, "The prefix micro- in microwave is not meant to suggest a wavelength in the micrometer range. It indicates that microwaves are "small", compared to the radio waves used prior to microwave technology, in that they have shorter wavelengths", so no, mi...
- Thu Oct 18, 2018 5:29 pm
- Forum: Photoelectric Effect
- Topic: Equation units
- Replies: 4
- Views: 443
Re: Equation units
We need to use kg because in the photoelectric effect equation: K = E - 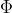 , Kinetic energy is measured in Joules (J) and one Joule is equal to 1 kgm2/s2
, Kinetic energy is measured in Joules (J) and one Joule is equal to 1 kgm2/s2
- Thu Oct 18, 2018 5:19 pm
- Forum: Photoelectric Effect
- Topic: 1B.25
- Replies: 2
- Views: 350
Re: 1B.25
Yes, using the Heisenbergs Uncertainty Principle equation,  p x
p x  X
X  h/4
h/4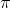 ,
,
you should get something like (note: don't forget to convert 350pm to meters first!):
 p
p  (6.626 x 10-34)/(4
(6.626 x 10-34)/(4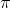 (3.5 x 10-10)(9.109 x 10-31))
(3.5 x 10-10)(9.109 x 10-31))
which gives you 1.65 x 105m/s
you should get something like (note: don't forget to convert 350pm to meters first!):
which gives you 1.65 x 105m/s
- Thu Oct 11, 2018 4:49 pm
- Forum: Properties of Light
- Topic: Homework Question 3; 6th Edition
- Replies: 2
- Views: 150
Re: Homework Question 3; 6th Edition
I was confused as well when I first read this. After looking a few things up, I believe it just means a change in energy. So, in the case of this question, because of the equation: E = hv, if frequency decreases, energy decreases as well since they are proportionally related.
- Thu Oct 11, 2018 4:41 pm
- Forum: Properties of Electrons
- Topic: Homework Questions Ch1 #7
- Replies: 2
- Views: 275
Re: Homework Questions Ch1 #7
For this question we're given the frequency and told to find the wavelength. In order to do this, we can use the equation: \lambda (wavelength) x \nu (frequency) = c(speed of light) plugging in what we have from part a of the question, this would give us: \lambda (7.1 x 10 14 )Hz = 3.00 x 10 8 m/s w...
- Thu Oct 11, 2018 4:28 pm
- Forum: Properties of Electrons
- Topic: Series and Wavelengths
- Replies: 2
- Views: 226
Series and Wavelengths
If the ultraviolet spectrum of an atom correlates to the Lyman series (n=1), do the other types of wavelengths (x-rays, visible light, etc) also correlate to other series (Balmer series, Paschen series, etc)?
- Thu Oct 04, 2018 8:40 pm
- Forum: Significant Figures
- Topic: Homework Regarding Avogadros Number
- Replies: 4
- Views: 331
Re: Homework Regarding Avogadros Number
It is safest to go to 6.022, however it depends on how many significant figures you're given in the original question. For example, if you were given 5 significant figures in the original question, you would probably want to go to at least the 5th significant figure in Avogadro's number (6.0221). Wh...
- Thu Oct 04, 2018 8:34 pm
- Forum: Significant Figures
- Topic: Limiting Reactants
- Replies: 6
- Views: 632
Re: Limiting Reactants
When looking for the limiting reactant, you should actually look at the moles and the ratio of the moles rather than the mass. Looking at the mass can be misleading because elements vary in mass. For example, if you given 13g of CH 4 and 30g of O 2 for the chemical reaction: CH 4 + 2O 2 --> CO 2 + 2...
- Thu Oct 04, 2018 8:14 pm
- Forum: Significant Figures
- Topic: Regarding the Homework
- Replies: 2
- Views: 204
Re: Regarding the Homework
After reading a few other posts in Chemistry Community, I'm pretty sure that it is okay to round a little while you're writing down your work for the homework problem, within good reason though. However, your final answer should follow significant figure rules. For example, if you're calculating the...

