Search found 66 matches
- Fri Mar 15, 2019 8:55 pm
- Forum: Reaction Mechanisms, Reaction Profiles
- Topic: Pre-equilibrium Approach
- Replies: 2
- Views: 628
Pre-equilibrium Approach
By the pre-equilibrium approach, we relate the rate laws that might contain intermediates by the equilibrium constant, correct? What happens if a reactant is a solid or liquid? How do we account for them then?
- Wed Mar 13, 2019 8:53 pm
- Forum: Arrhenius Equation, Activation Energies, Catalysts
- Topic: Arrhenius Equation and Collision Theory
- Replies: 1
- Views: 246
Arrhenius Equation and Collision Theory
How does the Arrhenius equation relate to collision theory, especially in regards to the Boltzmann distribution?
- Wed Mar 13, 2019 8:40 pm
- Forum: Entropy Changes Due to Changes in Volume and Temperature
- Topic: P-T Graphs
- Replies: 2
- Views: 501
P-T Graphs
Do we need to know how to use and draw P-T graphs?
- Wed Mar 13, 2019 8:25 pm
- Forum: Third Law of Thermodynamics (For a Unique Ground State (W=1): S -> 0 as T -> 0) and Calculations Using Boltzmann Equation for Entropy
- Topic: Calculating the Number of Orientations Mathematically
- Replies: 1
- Views: 420
Calculating the Number of Orientations Mathematically
How do we calculate the number of possible orientations or states mathematically when finding the degeneracy? In other words, if degeneracy W = x^(n), where x= number of possible orientations or states and n= # of particles, how do we mathematically find x?
- Wed Mar 06, 2019 10:24 am
- Forum: First Order Reactions
- Topic: Pseudo-First-Order Reaction
- Replies: 5
- Views: 545
Pseudo-First-Order Reaction
How do we know if a reaction can be treated as a pseudo-first-order reaction?
- Wed Mar 06, 2019 10:15 am
- Forum: Method of Initial Rates (To Determine n and k)
- Topic: Order of a Reaction
- Replies: 1
- Views: 296
Order of a Reaction
Is using the experimental data in rate ratios the only way to determine the order of a reaction? Is there another way to determine the order?
- Tue Mar 05, 2019 1:35 am
- Forum: General Rate Laws
- Topic: Rate Laws and Temperature
- Replies: 4
- Views: 449
Rate Laws and Temperature
How do rate laws relate to temperature in regards to equations?
- Wed Feb 27, 2019 11:22 pm
- Forum: Balancing Redox Reactions
- Topic: Basic Solutions
- Replies: 5
- Views: 475
Re: Basic Solutions
For basic solutions, you first balance the O by using H2O. Then, you balance H by adding H2O to the side of the half reaction that needs H and adding OH- to the other side.
- Wed Feb 27, 2019 11:18 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Redox Reactions and Cell Potentials
- Replies: 2
- Views: 296
Re: Redox Reactions and Cell Potentials
Since standard electric potential is an intensive property, it stays the same when the half reaction is balanced.
- Wed Feb 27, 2019 11:14 pm
- Forum: Balancing Redox Reactions
- Topic: Half Reactions in a Basic Solution
- Replies: 4
- Views: 413
Re: Half Reactions in a Basic Solution
To balance a half reaction in basic solution, you first add H2O to the side with the least amount of oxygen (let's call this side 1) to balance the oxygen. Then, you add H2O on the opposite side (side 2) and add OH- back to the original side (side 1) to balance the H.
- Thu Feb 21, 2019 12:36 am
- Forum: Balancing Redox Reactions
- Topic: Oxidation Numbers
- Replies: 2
- Views: 245
Re: Oxidation Numbers
The oxidation number of hydrogen is +1 except when bonded to metals in binary compounds, which is when hydrogen would have an oxidation number of -1.
- Thu Feb 21, 2019 12:30 am
- Forum: Balancing Redox Reactions
- Topic: Oxidation Numbers
- Replies: 2
- Views: 245
Re: Oxidation Numbers
The oxidation number of oxygen (O) is 2-, except in H2O2 (O2 = 2-) and O2 (O2 = 2-), where O is -1.
- Thu Feb 21, 2019 12:24 am
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Gibbs Free Energy Meaning
- Replies: 6
- Views: 753
Gibbs Free Energy Meaning
What really is Gibbs Free Energy, and what does it mean for a negative Gibbs Free Energy to result in a spontaneous reaction?
- Tue Feb 12, 2019 8:10 pm
- Forum: Administrative Questions and Class Announcements
- Topic: DOWNLOAD SESSION WORKSHEETS HERE - Sun 7-9PM (Karen)
- Replies: 179
- Views: 24024
Re: DOWNLOAD SESSION WORKSHEETS HERE - Sun 7-9PM (Karen)
On worksheet 3 Intro to Thermodynamics, problem 7, what equations are we supposed to use? My friend used the information given to make a cross multiplying equation but got an answer of 51.23g instead of 51.14g. How are we supposed to approach this problem?
- Tue Feb 12, 2019 9:29 am
- Forum: Third Law of Thermodynamics (For a Unique Ground State (W=1): S -> 0 as T -> 0) and Calculations Using Boltzmann Equation for Entropy
- Topic: Thermodynamic and Statistical Property
- Replies: 2
- Views: 276
Re: Thermodynamic and Statistical Property
LorenzoDuvergne3I wrote:Where is this discussed in the textbook?
This is from lecture notes.
- Tue Feb 12, 2019 9:29 am
- Forum: Entropy Changes Due to Changes in Volume and Temperature
- Topic: Entropy Change by Volume and Pressure
- Replies: 2
- Views: 323
Entropy Change by Volume and Pressure
If a system changes its conditions by both volume and pressure, do we have to solve for the entropy for both volume and pressure, even if the entropy is the same value for both conditions, since volume and pressure are inversely proportional?
- Mon Feb 11, 2019 3:31 pm
- Forum: Third Law of Thermodynamics (For a Unique Ground State (W=1): S -> 0 as T -> 0) and Calculations Using Boltzmann Equation for Entropy
- Topic: Thermodynamic and Statistical Property
- Replies: 2
- Views: 276
Thermodynamic and Statistical Property
What does it mean that thermodynamic property means a "small" error in S and a statistical property means a "large" error in W in relation to the Boltzmann equation?
- Thu Feb 07, 2019 10:17 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: q= -w
- Replies: 8
- Views: 710
Re: q= -w
If a system has a net internal energy change of zero, then the energy transferred by work and heat are equal and opposite of each other.
- Thu Feb 07, 2019 10:13 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: Internal Energy, Enthalpy, and Heat
- Replies: 2
- Views: 316
Internal Energy, Enthalpy, and Heat
Can someone explain in their own words what the relationship between internal energy, enthalpy, and heat is?
- Thu Feb 07, 2019 10:10 pm
- Forum: Entropy Changes Due to Changes in Volume and Temperature
- Topic: Purpose of ln
- Replies: 2
- Views: 257
Re: Purpose of ln
The integral of a variable to the (-1) is natural log, which gives the natural log in the work equation for a reversible process.
- Sat Feb 02, 2019 5:30 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Heat Capactiy
- Replies: 5
- Views: 493
Re: Heat Capactiy
The heat required depends on the amount of substance, so heat capacity is an extensive property.
- Sat Feb 02, 2019 5:28 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Bond Angles
- Replies: 5
- Views: 544
Re: Bond Angles
Energy is required to break bonds (positive enthalpy change), while energy is released to form bonds (negative enthalpy change).
- Sat Feb 02, 2019 5:25 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Enthalpy Symbol
- Replies: 5
- Views: 3747
Re: Enthalpy Symbol
Delta H naught of a reaction means the reactions are given in their standard state.
- Mon Jan 21, 2019 2:03 pm
- Forum: Administrative Questions and Class Announcements
- Topic: DOWNLOAD SESSION WORKSHEETS HERE - Sun 7-9PM (Karen)
- Replies: 179
- Views: 24024
Re: DOWNLOAD SESSION WORKSHEETS HERE - Sun 7-9PM (Karen)
On Worksheet 2, why is the concentration of CO3 the same as Ka2 for problem 4b?
- Mon Jan 21, 2019 1:48 pm
- Forum: Administrative Questions and Class Announcements
- Topic: DOWNLOAD SESSION WORKSHEETS HERE - Sun 7-9PM (Karen)
- Replies: 179
- Views: 24024
Re: DOWNLOAD SESSION WORKSHEETS HERE - Sun 7-9PM (Karen)
Did anyone happen to get around 0.077g for #2 on the acid-base review worksheet? The key says 0.10g, so I am unsure whether Karen rounded or I did the question incorrectly. I did the same thing at first. Make sure you use the initial concentration of HCOOH and not the equilibrium concentration of H...
- Mon Jan 21, 2019 1:37 pm
- Forum: Administrative Questions and Class Announcements
- Topic: DOWNLOAD SESSION WORKSHEETS HERE - Sun 7-9PM (Karen)
- Replies: 179
- Views: 24024
Re: DOWNLOAD SESSION WORKSHEETS HERE - Sun 7-9PM (Karen)
For number 7 on worksheet 1, why does high temperature favor the formation of bromine atoms if we don't know if the reaction is endothermic or exothermic?
- Fri Jan 18, 2019 1:38 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Equilibrium Arrow
- Replies: 7
- Views: 1048
Equilibrium Arrow
Do only reactions using weak acids and bases use an equilibrium arrow?
- Wed Jan 16, 2019 11:39 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Tricks for ICE tables
- Replies: 5
- Views: 508
Re: Tricks for ICE tables
If the problem is asking you to solve for the pH, pOH, or concentration of a reaction involving a weak acid or base, you use an ICE table because you can't assume 100% dissociation. You can tell if the reaction involves a weak acid by looking at the molecules or by the acidic constant if it is less ...
- Tue Jan 15, 2019 12:25 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Activity
- Replies: 2
- Views: 203
Activity
What is activity?
- Wed Jan 09, 2019 6:54 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Stability and Equilibrium Reactions
- Replies: 1
- Views: 172
Stability and Equilibrium Reactions
How do you tell if one equilibrium reaction is more stable than another?
- Tue Jan 08, 2019 7:42 pm
- Forum: Ideal Gases
- Topic: Activity
- Replies: 3
- Views: 276
Activity
What does 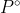 mean in the equation for activity?
mean in the equation for activity?
- Mon Jan 07, 2019 2:06 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Calculating Equilibrium Concentrations
- Replies: 3
- Views: 253
Calculating Equilibrium Concentrations
How do we calculate the equilibrium concentrations of the products and reactants? Will they be given?
- Fri Dec 07, 2018 5:23 pm
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: Coordination Compound Formation
- Replies: 1
- Views: 116
Coordination Compound Formation
Can someone explain to me how coordination compounds form, especially how transition metals are able to accept electron pairs from ligands?
- Fri Dec 07, 2018 5:21 pm
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: Iron in Myoglobin
- Replies: 2
- Views: 255
Iron in Myoglobin
Why can iron in myoglobin form 6 bonds?
- Tue Dec 04, 2018 5:31 pm
- Forum: Properties & Structures of Inorganic & Organic Acids
- Topic: Naming Acids
- Replies: 1
- Views: 1592
Naming Acids
Is there a method to naming acids, or do we just need to memorize them?
- Thu Nov 29, 2018 11:32 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: bond angle
- Replies: 6
- Views: 524
Re: bond angle
Yes, the approximation of the bond angle is acceptable, so < 120 degrees works.
- Thu Nov 29, 2018 11:28 am
- Forum: Lewis Acids & Bases
- Topic: HF
- Replies: 5
- Views: 596
HF
Why is HF a weak acid?
- Wed Nov 28, 2018 10:17 pm
- Forum: Hybridization
- Topic: Delocalized Pi Bonding
- Replies: 3
- Views: 304
Delocalized Pi Bonding
Can someone explain what delocalized pi bonding is and when does it exist?
- Tue Nov 20, 2018 12:12 am
- Forum: Lewis Structures
- Topic: Polar vs Nonpolar molecules
- Replies: 7
- Views: 900
Re: Polar vs Nonpolar molecules
You check for electronegativity, dipole moments, and then molecular shape to determine is a molecule is polar or nonpolar.
- Mon Nov 19, 2018 4:37 pm
- Forum: Hybridization
- Topic: Sigma and Pi Bonds [ENDORSED]
- Replies: 12
- Views: 1093
Re: Sigma and Pi Bonds [ENDORSED]
Also, sigma bonds are made from hybridized orbitals and pi bonds are made from unhybridized orbitals.
- Mon Nov 19, 2018 4:31 pm
- Forum: Hybridization
- Topic: Hybridized vs. Unhybridized Orbitals
- Replies: 4
- Views: 4379
Re: Hybridized vs. Unhybridized Orbitals
If this helps, a sigma bond is made up of hybridized orbitals and a pi bond is made up of unhybridized orbitals
- Thu Nov 15, 2018 9:14 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Trigonal Planar
- Replies: 5
- Views: 481
Re: Trigonal Planar
In the VSEPR Model, all bonds are treated as equivalent, so no, double bonds (or any type of bonds in general) are not significant to the structure. We only account for the regions of electron density.
- Mon Nov 12, 2018 4:00 pm
- Forum: Dipole Moments
- Topic: Dipole Moments and Polar Molecules
- Replies: 2
- Views: 286
Dipole Moments and Polar Molecules
How do you calculate for the dipole moment of a molecule and use it to determine whether a molecule is polar or nonpolar?
- Mon Nov 12, 2018 1:08 pm
- Forum: Lewis Structures
- Topic: N2O Lewis Structure
- Replies: 1
- Views: 194
N2O Lewis Structure
For N2O, why is nitrogen the central atom if oxygen has the lower ionization energy?
- Fri Nov 09, 2018 1:21 am
- Forum: Dipole Moments
- Topic: London Forces
- Replies: 2
- Views: 307
London Forces
Why do London forces exist?
- Wed Nov 07, 2018 8:58 pm
- Forum: Resonance Structures
- Topic: Resonance Lewis Structures
- Replies: 3
- Views: 424
Resonance Lewis Structures
Are resonance structures only in regards to bond location? Do they apply to the positions of lone pair electrons too?
- Tue Nov 06, 2018 12:50 pm
- Forum: Lewis Structures
- Topic: Lewis Structure for NO2
- Replies: 3
- Views: 385
Lewis Structure for NO2
If oxygen has a lower ionization energy than nitrogen, why is nitrogen the central atom in the NO2 Lewis Structure? Is it to maintain symmetry?
- Fri Nov 02, 2018 1:55 pm
- Forum: Properties of Electrons
- Topic: De Broglie Formula vs. Speed of Light Formula
- Replies: 5
- Views: 699
Re: De Broglie Formula vs. Speed of Light Formula
Thanks everyone! I realized that the question involves copper metal, which has resting mass, so we have to use the De Broglie Formula.
- Fri Nov 02, 2018 10:22 am
- Forum: Properties of Electrons
- Topic: De Broglie Formula vs. Speed of Light Formula
- Replies: 5
- Views: 699
Re: De Broglie Formula vs. Speed of Light Formula
In the case of Question 3C on Test 2, how do we know when to used the De Broglie equation then?
- Fri Nov 02, 2018 2:56 am
- Forum: Properties of Electrons
- Topic: De Broglie Formula vs. Speed of Light Formula
- Replies: 5
- Views: 699
De Broglie Formula vs. Speed of Light Formula
How do you know when to use the De Broglie formula versus the speed of light formula?
- Wed Oct 31, 2018 9:21 pm
- Forum: Formal Charge and Oxidation Numbers
- Topic: Oxidation Numbers
- Replies: 3
- Views: 513
Oxidation Numbers
How do you know the oxidation number for an atom of an element?
- Fri Oct 26, 2018 1:36 am
- Forum: Lewis Structures
- Topic: Drawing Lewis Structures
- Replies: 3
- Views: 505
Drawing Lewis Structures
When drawing a Lewis Structure, how do you know specifically where to bond the atoms? What are the steps to drawing accurate Lewis Structures of atoms bonded together?
- Tue Oct 23, 2018 11:55 pm
- Forum: Heisenberg Indeterminacy (Uncertainty) Equation
- Topic: Uncertainty of an Electron's Velocity & Speed of Light
- Replies: 4
- Views: 499
Uncertainty of an Electron's Velocity & Speed of Light
Why is the Incorrect Atomic Model disproved because the uncertainty in the velocity of the electron is greater than the speed of light? What is important about the speed of light that disproves this model?
- Tue Oct 23, 2018 11:13 pm
- Forum: Bohr Frequency Condition, H-Atom , Atomic Spectroscopy
- Topic: Spectral Lines and Series
- Replies: 3
- Views: 399
Spectral Lines and Series
Why are spectral lines important? Why are the Balmer and Lyman series significant? I know the Balmer series represents visible light, and the Lyman series represents UV light, but why is that important?
- Wed Oct 17, 2018 4:56 pm
- Forum: Quantum Numbers and The H-Atom
- Topic: Quantum Number L [ENDORSED]
- Replies: 3
- Views: 378
Quantum Number L [ENDORSED]
What does orbital angular momentum show us, and why is it important?
- Wed Oct 17, 2018 4:51 pm
- Forum: Quantum Numbers and The H-Atom
- Topic: Principal Quantum Number (n)
- Replies: 3
- Views: 351
Principal Quantum Number (n)
What does the principal quantum number (n) refer to? Does it refer to shell size? Could someone give their own explanation of how the quantum numbers n, l, ml, and ms relate to each other, and their purpose in the atomic structure?
- Wed Oct 17, 2018 4:45 pm
- Forum: Quantum Numbers and The H-Atom
- Topic: Wavefunction of the Ground State of the Hydrogen Atom
- Replies: 1
- Views: 257
Wavefunction of the Ground State of the Hydrogen Atom
In the wavefunction of the ground state of the hydrogen atom, where  (r,
(r, 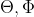 ) = Ne^(-r/a0), what does N represent? What purpose does N serve?
) = Ne^(-r/a0), what does N represent? What purpose does N serve?
- Wed Oct 10, 2018 10:22 pm
- Forum: *Particle in a Box
- Topic: Purpose of Particle in a Box
- Replies: 2
- Views: 1100
Purpose of Particle in a Box
What is the purpose of the particle in a box concept, and what does it tell us exactly?
- Wed Oct 10, 2018 7:21 pm
- Forum: Bohr Frequency Condition, H-Atom , Atomic Spectroscopy
- Topic: Meaning of Quanta/Quantized
- Replies: 2
- Views: 340
Meaning of Quanta/Quantized
Can someone explain what "quanta" and "quantized" means?
- Mon Oct 08, 2018 1:05 pm
- Forum: Limiting Reactant Calculations
- Topic: Maximum Product Produced
- Replies: 2
- Views: 330
Re: Maximum Product Produced
Additional Information: one mole of A is mixed with one mole of B. Update after asking a TA: Since the mole ratio between A and C is 2:3, that means 1 mole of A would yield 1.5 moles of C. The mole ratio between B and C is 1:3, which means 1 mole of B would yield 3 moles of C. Therefore, A is the li...
- Fri Oct 05, 2018 11:23 am
- Forum: Molarity, Solutions, Dilutions
- Topic: Water Added to Solution Placed in a New Flask
- Replies: 3
- Views: 615
Water Added to Solution Placed in a New Flask
5.00 g of KMnO4 is dissolved in a 150.00 mL flask of water. If 20.00 mL of this solution is removed and placed in a new 2nd 250.00 mL flask and filled with water, what is the concentration of the solution in the 2nd flask? Molar Masses: K (39.10 g/mol), Mn (54.94 g/mol), O (16.00 g/mol) How do you s...
- Fri Oct 05, 2018 10:07 am
- Forum: Limiting Reactant Calculations
- Topic: Maximum Product Produced
- Replies: 2
- Views: 330
Maximum Product Produced
In the balanced equation 2A + 1B ---> 3C, how do you find the maximum product produced?
*This is under Question 30 in the Limiting Reactant Calculations Audio-Visual Focus Topics Post-Assessment.*
*This is under Question 30 in the Limiting Reactant Calculations Audio-Visual Focus Topics Post-Assessment.*
- Fri Oct 05, 2018 10:05 am
- Forum: Limiting Reactant Calculations
- Topic: Compensating for Mole Ratios in Limiting Reactants
- Replies: 3
- Views: 443
Compensating for Mole Ratios in Limiting Reactants
In the chemical reaction 4NH3(g) + 5O2(g) ---> 4NO(g) + 6H2O(g), how do you find the limiting reactant if 21.4 g NH3 is reacted with 42.5 g of O2? How do you compensate for the mole ratio when calculating?
- Fri Oct 05, 2018 7:40 am
- Forum: Limiting Reactant Calculations
- Topic: Excess Reactant Calculation
- Replies: 2
- Views: 303
Excess Reactant Calculation
What are the steps to finding the excess of a reactant in a chemical reaction?
- Tue Oct 02, 2018 11:23 pm
- Forum: Balancing Chemical Reactions
- Topic: Mole Ratios & Stoichiometric Relations
- Replies: 2
- Views: 352
Mole Ratios & Stoichiometric Relations
Do stoichiometric relations and mole ratios describe the same relationship between substances? What are stoichiometric relations used for? Is it just another way to express a mole ratio?
- Mon Oct 01, 2018 7:25 pm
- Forum: Balancing Chemical Reactions
- Topic: Law of Conservation of Mass [ENDORSED]
- Replies: 7
- Views: 1075
Re: Law of Conservation of Mass [ENDORSED]
Yes, basically the total mass before a reaction is always equal to the total mass after. Also I liked this concept too: in chemical reactions, atoms are not created or destroyed; they are rearranged.

