Search found 75 matches
- Tue Mar 12, 2019 9:17 pm
- Forum: Kinetics vs. Thermodynamics Controlling a Reaction
- Topic: 15.53
- Replies: 1
- Views: 421
Re: 15.53
Mechanism C wouldn't agree with the proposed rate law for the same reason that mechanism A wouldn't: the rate law would include CO. Having a reverse reaction in step 1 means that the reactants used to produce NO 3 can be used in the rate law. Thus, for (c), rate = k[NO 2 ] 2 [CO]. But, again, this d...
- Tue Mar 12, 2019 9:02 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: value of equilibrium constant
- Replies: 1
- Views: 202
Re: value of equilibrium constant
The value of the equilibrium constant is only affected by a change in temperature. However, the position of equilibrium itself (i.e. the state when both reactants and products are present in concentrations that tend not to change over time) is affected by many factors. These include change in concen...
- Tue Mar 12, 2019 8:54 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Ice melting
- Replies: 4
- Views: 743
Re: Ice melting
Ice melting is a spontaneous process because the order of the water molecules is interrupted, meaning that entropy increases (+∆S). Even though it requires energy (endothermic, +∆H), the reaction usually occurs in high temperatures, resulting in -∆G. The combustion of a natural gas is spontaneous be...
- Tue Mar 12, 2019 3:01 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Van't Hoff Equation Test #2
- Replies: 1
- Views: 331
Re: Van't Hoff Equation Test #2
Yes, in the test, K w was K 1 . Once you determine the K 2 value, you can use the rule: K = [H + ][OH - ]. This means you can square root the K 2 value to get [H + ], which you can then plug in pH = -log[H + ]. The pH value you calculate will be the new pH value for neutral solutions. For example, s...
- Tue Mar 12, 2019 12:51 pm
- Forum: Calculating Standard Reaction Entropies (e.g. , Using Standard Molar Entropies)
- Topic: Concept of molar entropy
- Replies: 2
- Views: 583
Re: Concept of molar entropy
When one substance has a lower molar entropy than another, it means that substance is comparatively more ordered (less disorder). This further indicates that more heat is needed to make it more disordered than the other substance. For example, ice (solid H 2 O) has lower molar entropy than water (li...
- Tue Mar 12, 2019 12:43 pm
- Forum: Second Order Reactions
- Topic: amounts of reactant and its affect on rate
- Replies: 6
- Views: 573
Re: amounts of reactant and its affect on rate
Yes, because the rate of that second order reaction is proportional to the squared value of reactant A. However, remember that this applies to reactions that only have one reactant. The overall order of the reaction would be different if there were more than one reactants.
- Tue Mar 12, 2019 12:36 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Strong Acid/Base Concentration vs. Weak Acid/Base Concentration
- Replies: 2
- Views: 313
Re: Strong Acid/Base Concentration vs. Weak Acid/Base Concentration
For strong acids, the concentration of H + ions generally equals the concentration of the strong acid. For strong bases, the concentration of OH - ions generally equals the concentration of the strong base. However, for weak acids and bases, their respective concentrations of H + and OH - ions don't...
- Tue Mar 12, 2019 12:23 pm
- Forum: Reaction Mechanisms, Reaction Profiles
- Topic: rxn mechanisms
- Replies: 3
- Views: 402
Re: rxn mechanisms
Reaction mechanisms tell us the order and number of elementary steps that occur to form products. They also tell us whether or not reaction intermediates are formed then consumed. The slowest step in the mechanism, which is usually indicated, determines the rate of the reaction.
- Wed Feb 27, 2019 11:41 pm
- Forum: Balancing Redox Reactions
- Topic: Basic Solutions
- Replies: 5
- Views: 478
Re: Basic Solutions
In basic solutions, the OH- ions tend to be on the reactants side in the oxidation half-reaction. Then, the OH- ions tend to be on the products side in the reduction half-reaction.
- Wed Feb 27, 2019 11:36 pm
- Forum: Balancing Redox Reactions
- Topic: oxidizing agent and reducing agent
- Replies: 15
- Views: 1377
Re: oxidizing agent and reducing agent
The oxidizing agent is the molecule that has the atom whose oxidation state decreases, due to gaining electrons (reduction). Meanwhile, the reducing agent is the molecule that has the atom whose oxidation state increases, due to the loss of electrons (oxidation).
- Fri Feb 22, 2019 9:39 am
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Equations of delta G
- Replies: 4
- Views: 540
Re: Equations of delta G
Don’t forget that there is also this equation: ∆G = ∆G° + RTlnQ A lot of homework questions about Gibbs free energy can only be answered with this. It’s generally used when given a temperature value, a K value, and concentations/partial pressures of reactants and products to calculate Q.
- Thu Feb 21, 2019 4:30 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: 6th edition, 6L3
- Replies: 1
- Views: 238
Re: 6th edition, 6L3
E values of half reactions can be used to figure out which one is reduced and oxidized. A more positive reduction potential will tend to be reduced (i.e. gain electrons), while a comparatively more negative reduction potential will tend to be oxidized (i.e. lose electrons).
- Thu Feb 21, 2019 4:24 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Reduction Potentials
- Replies: 3
- Views: 531
Re: Reduction Potentials
Reduction potentials can be calculated by comparing the electron transfer of the cell (half reaction) with respect to the Standard Hydrogen Electrode under the conditions of 1M solution, 1 atm, and 25°C (298K). Reduction potentials can also be calculated by using or rearranging this equation: E° cel...
- Thu Feb 21, 2019 4:20 pm
- Forum: Balancing Redox Reactions
- Topic: Oxidation States
- Replies: 10
- Views: 1007
Re: Oxidation States
You can use Toolbox K.1 in the 6th edition of the textbook to determine the basic oxidation states. As for metals, it usually depends on the other atoms in the molecule they're in. This is because transition metals can have a range of oxidation states, like the ones in the attached image (the ones i...
- Thu Feb 21, 2019 4:08 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Flow of electrons
- Replies: 4
- Views: 472
Re: Flow of electrons
If by anode, you mean the electrode where oxidation occurs, and by cathode, you mean the electrode where the reduction takes place, then yes, electrons always flow from anode to cathode. This can be confusing depending on the cell (voltaic or electrolytic) in which the reaction takes place.
- Thu Feb 21, 2019 3:57 pm
- Forum: Thermodynamic Systems (Open, Closed, Isolated)
- Topic: Midterm #1 14B
- Replies: 17
- Views: 2209
Re: Midterm #1 14B
What TA's review sessions did y'all find to be the most helpful? Because I've gone to a few where I feel more lost when I leave then helped. From the review sessions for the midterm, I found those by Morris, Nathan, and Ronald the most helpful because they were fun and covered the material in a way...
- Thu Feb 21, 2019 3:34 pm
- Forum: Balancing Redox Reactions
- Topic: Finding Charge
- Replies: 3
- Views: 399
Re: Finding Charge
If by "charge is not given," you mean that the molecule has a neutral charge, then the charge would be 0. For example, H 2 O is a neutral molecule; it has a net charge of 0. Based on the charges from the periodic table, oxygen usually has a 2- charge while hydrogen usually has a + charge. ...
- Thu Feb 21, 2019 12:36 pm
- Forum: Balancing Redox Reactions
- Topic: Writing Half Reactions
- Replies: 2
- Views: 313
Re: Writing Half Reactions
When writing half reactions for redox reactions in acidic solution, the only acceptable additions are H 2 O, H + , and electrons. For example, balancing this reduction reaction: Cr 2 O 7 2- → Cr 3+ 1. Balance elements in the equation other than oxygen and hydrogen. so, Cr 2 O 7 2- → 2 Cr 3+ 2. Balan...
- Thu Feb 21, 2019 12:15 pm
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: salt bridge
- Replies: 4
- Views: 563
Re: salt bridge
The salt bridge allows ion transfer between two half reactions by providing an electrical connection within which ions can flow. This keeps both solutions at a neutral state, so electron flow can continue and be measurable through a voltmeter. Salt bridges usually contain an unreactive electrolyte t...
- Thu Feb 21, 2019 12:11 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Negative ∆G means spontaneous reaction?
- Replies: 5
- Views: 7091
Re: Negative ∆G means spontaneous reaction?
A reaction is spontaneous if it occurs without help or work. A reaction with negative ∆G° is spontaneous, since ∆G° usually results from negative ∆H° and positive ∆S°. The latter means that disorder of the universe increases, which always applies to spontaneous processes. However, remember that all ...
- Thu Feb 21, 2019 12:01 pm
- Forum: Work, Gibbs Free Energy, Cell (Redox) Potentials
- Topic: Which ion will be oxidized/reduced?
- Replies: 1
- Views: 221
Re: Which ion will be oxidized/reduced?
One can tell which ion will be oxidized or reduced based on their standard reduction potentials, E°. A solution with a more positive reduction potential will tend to gain electrons (i.e. to be reduced by oxidizing the new species). Meanwhile, a solution with a more negative reduction potential will ...
- Thu Feb 21, 2019 11:55 am
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Pt as an electrode
- Replies: 4
- Views: 639
Re: Pt as an electrode
Being an inert and unreactive electrode means that it will not give or take any electrons during the reaction. This leaves the reduction and oxidation reactions to the reactants in question.
- Thu Feb 21, 2019 11:50 am
- Forum: Work, Gibbs Free Energy, Cell (Redox) Potentials
- Topic: Free Energy
- Replies: 5
- Views: 513
Re: Free Energy
The change in Gibbs free energy is a state function, i.e. it is additive. Dr. Lavelle showed in lecture that ∆G°rxn = ΣnGf°(products) - ΣnGf°(reactants)
- Thu Feb 21, 2019 11:43 am
- Forum: Balancing Redox Reactions
- Topic: k from lnK
- Replies: 5
- Views: 4004
Re: k from lnK
To exhibit the answers above, here's an example: if lnK = 0, then K = e0, making K = 1.
- Thu Feb 21, 2019 11:39 am
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Anode v. Cathode
- Replies: 9
- Views: 986
Re: Anode v. Cathode
One way to remember the left-hand (anode) and right-hand (cathode) electrodes is the acronym AN OIL RIG CAT. This stands for Anode for Oxidation Is Losing elections, while Reduction Is Gaining elections in the Cathode.
- Thu Feb 21, 2019 11:32 am
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Gibbs Free Energy vs Entropy
- Replies: 4
- Views: 800
Re: Gibbs Free Energy vs Entropy
In addition to the answers above, Gibbs free energy also allows you to determine whether or not a reaction is likely to occur. Based on the ∆G°= ∆H°- T∆S, negative enthalpy and positive entropy will result to a negative Gibbs free energy, which favors the forward reaction. When enthalpy is positive ...
- Thu Feb 21, 2019 11:26 am
- Forum: Balancing Redox Reactions
- Topic: Week 7 Homework
- Replies: 15
- Views: 1253
Re: Week 7 Homework
I answered a bunch of Gibbs Free Energy problems, and a couple of electrochemistry questions because it was taught in lecture yesterday.
- Tue Jan 15, 2019 3:04 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Kc, Kw, and pKw
- Replies: 4
- Views: 9717
Re: Kc, Kw, and pKw
Kc is a reaction's equilibrium constant. 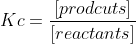
Kw is the ionization constant of water. Kw = [H3O+][OH-] = 1.0 x 10-14
pKw is just the negative log of water's ionization constant. pKw = -log(pKw)
Kw is the ionization constant of water. Kw = [H3O+][OH-] = 1.0 x 10-14
pKw is just the negative log of water's ionization constant. pKw = -log(pKw)
- Tue Jan 15, 2019 2:41 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Question on 12.41
- Replies: 3
- Views: 264
Re: Question on 12.41
I believe both C5H5N and NH3 are in table 12.2. As for the anions, you can find their pKb values by subtracting the pKa values of their parent acids (HF and CH3COOH), found in table 12.1, from 14 (which is pKw). The lower the pKb value, the stronger the base is.
- Tue Jan 15, 2019 2:24 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: ICE Tables [ENDORSED]
- Replies: 11
- Views: 2441
Re: ICE Tables [ENDORSED]
ICE tables are often used when you're asked to find equilibrium concentrations, and when you're given initial concentrations and a Kc value. However, you can also be asked to do the other way around: find initial concentrations from equilibrium concentrations and a Kc value. So, no, the concentratio...
- Tue Jan 15, 2019 2:12 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Q vs. K
- Replies: 5
- Views: 509
Re: Q vs. K
Can Q fluctuate around K so that it could be higher at one point and lower at another point? I believe that Q can fluctuate around K, depending on the specific given time you measure Q. For instance, if you measure Q a few seconds after products are added to the system, Q will be greater than K (th...
- Tue Jan 15, 2019 2:01 pm
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Part 4 Post-Module Question
- Replies: 4
- Views: 1287
Re: Part 4 Post-Module Question
The delta H values indicate whether the reaction is endothermic or exothermic. When delta H is positive, the reaction is endothermic, simply meaning that heat is in the reactants side. When delta H is negative, the reaction is exothermic, simply meaning that heat is in the products side. Thus, in a)...
- Tue Jan 15, 2019 1:53 pm
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: positive vs. negative delta H
- Replies: 2
- Views: 44481
Re: positive vs. negative delta H
Positive delta H is endothermic because the system absorbed heat, which means that there is more energy in the products side. This results to a positive difference in H, when H of reactants is subtracted from H of products. On the other hand, negative delta H is exothermic because the system release...
- Thu Dec 06, 2018 5:47 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Polarizing
- Replies: 1
- Views: 223
Re: Polarizing
The more negative the charge of an anion (the greater the number of electrons compared to the number of protons) and bigger its ionic radius, the more polarizable it is. For example P 3- is more polarizable than Cl - . The more positive the charge of a cation (the greater the number of protons compa...
- Thu Dec 06, 2018 5:35 pm
- Forum: Amphoteric Compounds
- Topic: Amphoteric Compounds
- Replies: 1
- Views: 328
Re: Amphoteric Compounds
A compound is amphoteric if it is able to react as both a Lewis base (lone pair donor) and as a Lewis acid (lone pair acceptor). Also, metal oxides (Na 2 O, CaO) react with water to form strong bases, while nonmetal oxides (ex. CO 2 , SO 2 ) react with water to form acids. As for As 2 O 3 and Bi 2 O...
- Tue Dec 04, 2018 1:25 am
- Forum: Naming
- Topic: H20 as ligand
- Replies: 2
- Views: 178
Re: H20 as ligand
OH 2 is often used to make it clear that it is the oxygen (not the hydrogen) that is bonded to the central atom. People can overlook that and make mistakes, especially when drawing Lewis structures. Other than that, as long as you know that the bond constitutes electrons that belong to oxygen, I bel...
- Tue Dec 04, 2018 1:20 am
- Forum: Calculating pH or pOH for Strong & Weak Acids & Bases
- Topic: Is there a difference between concentration and molarity?
- Replies: 2
- Views: 12903
Re: Is there a difference between concentration and molarity?
Yes, there is a difference between concentration and molarity. As you indicated with "concentration as g/L of Kg/L," concentration is the ratio of the amount of solute per amount of solution. Meanwhile, molarity is a unit of concentration that relates the amount of moles of a solute per li...
- Tue Dec 04, 2018 1:13 am
- Forum: Hybridization
- Topic: Hybridization
- Replies: 11
- Views: 1302
Re: Hybridization
In terms of electron geometry, sp 3 d generally corresponds with the trigonal bipyramidal shape, which has a VSEPR formula of AX 5 . It can definitely differ with regards to molecular geometry though, as stated by the prior answers. Nonetheless, sp 3 d hybridization indicates that five hybrid orbita...
- Tue Dec 04, 2018 12:47 am
- Forum: Air Pollution & Acid Rain
- Topic: Acid Rain Equations
- Replies: 1
- Views: 447
Re: Acid Rain Equations
One equation include sulfur: S + O 2 → SO 2 2SO 2 + O 2 → 2SO 3 SO 3 + H 2 O → H 2 SO 4 Sulphur, which comes from burning of coal, reacts with oxygen in the air to form sulfur dioxide (an acidic oxide). Sulfur dioxide is then oxidized, forming sulfur trioxide. The sulfur trioxide reacts with either ...
- Sun Nov 25, 2018 2:48 pm
- Forum: Hybridization
- Topic: SeF3+
- Replies: 5
- Views: 8433
Re: SeF3+
For SeF3+, I believe the molecular geometry is trigonal pyramid, AX3E. The central atom is Se, each F is single bonded to Se, then Se would have a lone pair as well. You can calculate the formal charge of each atom to check if this is correct. Hope this helps.
- Sun Nov 25, 2018 12:11 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Shapes Not covered in Lecture
- Replies: 10
- Views: 1142
Re: Shapes Not covered in Lecture
I believe that it would be good to know those shapes even if they weren't covered in lecture, since some VSEPR shapes (like T-shaped) are in the homework questions on the outline.
- Sun Nov 25, 2018 12:06 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Polarity
- Replies: 6
- Views: 573
Re: Polarity
A VSEPR model shape is not always polar, even if the central atom has lone pairs. An example of a shape where lone pairs are symmetrical and cancel out each other's charges is square planar, AX 4 E 2 . This is because the lone pairs are in axial position away from each other (i.e. 180° away, on oppo...
- Sun Nov 25, 2018 11:57 am
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Bonds
- Replies: 2
- Views: 176
Re: Bonds
A single bond is always a sigma bond.
A double bond is always one sigma bond and one pi bond.
A triple bond is always one sigma bond and two pi bonds.
A double bond is always one sigma bond and one pi bond.
A triple bond is always one sigma bond and two pi bonds.
- Tue Nov 20, 2018 4:42 pm
- Forum: Hybridization
- Topic: Pi and Sigma Bonds
- Replies: 4
- Views: 470
Re: Pi and Sigma Bonds
I've always thought of sigma bonds as "head on collisions" between two orbitals, while pi bonds are just sideways overlaps between two orbitals. Once orbitals have "collided," they can't collide anymore, i.e. once a sigma bond has been formed between two atoms/molecules, another ...
- Tue Nov 20, 2018 4:34 pm
- Forum: Hybridization
- Topic: Homework Question 7th Edition 2F.15
- Replies: 2
- Views: 304
Re: Homework Question 7th Edition 2F.15
I was confused about that question and s-character as well since we haven't really directly discussed it in depth, but a chem mod explains it pretty well here: viewtopic.php?t=1255
- Tue Nov 20, 2018 4:02 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: VSEPR Notation
- Replies: 2
- Views: 233
Re: VSEPR Notation
I believe that VSEPR notation will be on the test because many of the homework problems ask us to write the VSEPR formulas of various species. VSEPR formulas are relatively easy to determine, so knowing both ways (the one taught in class and your own method) definitely won't hurt you!
- Tue Nov 20, 2018 3:56 pm
- Forum: Hybridization
- Topic: Regions of electron density
- Replies: 6
- Views: 752
Re: Regions of electron density
Yes, I also believe that's correct, even if one of the electron densities isn't involved in a bond with another atom or molecule.
- Tue Nov 20, 2018 3:42 pm
- Forum: Hybridization
- Topic: Hybrid Orbital Energy
- Replies: 1
- Views: 158
Hybrid Orbital Energy
Is the energy of a hybrid orbital equal to the average of the energies of the orbitals that make it up? For example, is the energy of an sp3 hybrid orbital equal the average of the energies of one s orbital and three p orbitals?
- Tue Nov 20, 2018 3:37 pm
- Forum: Sigma & Pi Bonds
- Topic: What makes up a sigma bond?
- Replies: 1
- Views: 255
What makes up a sigma bond?
Based on Monday’s lesson (11/19) on hybridization, is it safe to assume that at least one hybrid orbital constitutes a sigma bond?
- Mon Nov 12, 2018 10:11 pm
- Forum: Ionic & Covalent Bonds
- Topic: London Van Der Waals Force?
- Replies: 5
- Views: 540
Re: London Van Der Waals Force?
London Van Der Waals Forces are fluctuating dipoles that result from fluctuating electron distributions, due to electrostatic interactions between molecules. They are always present and attractive. Yes, according to Dr. Lavelle's lecture, they are synonymous to dispersion and induced dipole-induced ...
- Mon Nov 12, 2018 9:53 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Molecular Shape Affects Strength of Interaction
- Replies: 3
- Views: 210
Re: Molecular Shape Affects Strength of Interaction
Do rod-shaped molecules induce dipole moments in spherical molecules?
- Mon Nov 12, 2018 9:32 pm
- Forum: Dipole Moments
- Topic: Interaction Potential Energy
- Replies: 3
- Views: 235
Re: Interaction Potential Energy
Will we use the potential energy equation in test 3 and/or the final exam?
- Tue Oct 23, 2018 9:44 pm
- Forum: Bohr Frequency Condition, H-Atom , Atomic Spectroscopy
- Topic: Number 15 for chapter 1
- Replies: 1
- Views: 195
Re: Number 15 for chapter 1
Hi, I attached an image that shows how the Rydberg equation is derived. Hope it helps. Also, I do think that such questions will be on the exam because it shows understanding of the concepts, but prof said that he doesn't like using that equation. He's rather us calculate frequency (nu) from ΔE dire...
- Tue Oct 23, 2018 9:16 pm
- Forum: Student Social/Study Group
- Topic: Questions about Heisenberg
- Replies: 5
- Views: 620
Re: Questions about Heisenberg
Yes, I'm sure. Check this out too if you're still uncertain: viewtopic.php?f=19&t=34622&p=113448
- Tue Oct 23, 2018 9:06 pm
- Forum: Student Social/Study Group
- Topic: Experiment supporting de Broglie's
- Replies: 3
- Views: 410
Re: Experiment supporting de Broglie's
According to prof Lavelle's "Wave Properties of Electrons and the DeBroglie Equation" module, that experiment supports the hypothesis because the only time a diffraction pattern can be observed is when whatever is being used is acting like a wave. In this case, light diffracts as it become...
- Tue Oct 23, 2018 8:56 pm
- Forum: Student Social/Study Group
- Topic: Questions about Heisenberg
- Replies: 5
- Views: 620
Re: Questions about Heisenberg
Delta v would be 0.2 because 23 +/- 0.1 indicates that possible values lie within 22.9 and 23.1
Since 23.1 - 22.9 = 0.2, delta v is 0.2
Since 23.1 - 22.9 = 0.2, delta v is 0.2
- Tue Oct 23, 2018 8:53 pm
- Forum: Wave Functions and s-, p-, d-, f- Orbitals
- Topic: 2.19 (b)
- Replies: 3
- Views: 377
Re: 2.19 (b)
The f subshell corresponds to l=3, so possible m values would be -3, -2, -1, 0, 1, 2, and 3. Not sure why the solutions manual would say otherwise though.
- Tue Oct 23, 2018 8:49 pm
- Forum: Photoelectric Effect
- Topic: Example 1.5 6th edition
- Replies: 2
- Views: 357
Re: Example 1.5 6th edition
I took the test today and that conversion was not in it. Nonetheless, it's an important conversion to keep in mind.
- Tue Oct 23, 2018 8:41 pm
- Forum: Heisenberg Indeterminacy (Uncertainty) Equation
- Topic: Test 2 and Homework Problems
- Replies: 6
- Views: 666
Re: Test 2 and Homework Problems
Brevin Hensley 3F wrote:Since Test 2 is suppose to be all quantum material up to and including quantum numbers, does this mean electron material such as configurations and electron affinity will not be on the test?
Yes, configurations and electron affinity will not be on the test.
- Tue Oct 23, 2018 7:46 pm
- Forum: *Particle in a Box
- Topic: Test 2
- Replies: 9
- Views: 1379
Re: Test 2
You must know about the quantum numbers and their possible values. So yes, test 2 covers material about orbitals, but I don't think that should be your biggest concern.
- Tue Oct 23, 2018 7:40 pm
- Forum: Heisenberg Indeterminacy (Uncertainty) Equation
- Topic: Test 2 and Homework Problems
- Replies: 6
- Views: 666
Re: Test 2 and Homework Problems
Abby De La Merced 3F wrote:Does anyone know if the test is strictly problem based or will there be conceptual questions as well?
In the test I took at section today, both types of questions were present, so I suggest studying/practicing both.
- Tue Oct 23, 2018 7:36 pm
- Forum: DeBroglie Equation
- Topic: DeBroglie Equation Post Assessment
- Replies: 3
- Views: 341
Re: DeBroglie Equation Post Assessment
With this problem, use the deBroglie Equation: λ = h/p, where p is momentum = masselectron * velocity. You can rearrange the equation to calculate the velocity: v = h/(λ * masselectron)
- Tue Oct 23, 2018 7:32 pm
- Forum: Bohr Frequency Condition, H-Atom , Atomic Spectroscopy
- Topic: 1A 15 7th edition help
- Replies: 1
- Views: 273
Re: 1A 15 7th edition help
Considering that it's from the ultraviolet spectrum, it's evident that the line is from the Lyman series. The lines in this series result from hydrogen's electron going from n≥2 to n=1. Thus, in the problem, n2 in the Rydberg equation would be 1. Hope this helps you get started!
- Tue Oct 23, 2018 7:17 pm
- Forum: Quantum Numbers and The H-Atom
- Topic: Angular Momentum Quantum Numbers
- Replies: 3
- Views: 482
Re: Angular Momentum Quantum Numbers
The "l=n-1" indicates that the shell (principal quantum number, n) limits the number of subshells (angular momentum quantum number, l) that can be inside it. Say n=2, thus the only possible l values are 0 and 1, which further means that the only subshells within the n=2 shell are s and p r...
- Tue Oct 23, 2018 7:03 pm
- Forum: Properties of Light
- Topic: Test 2 Equations
- Replies: 14
- Views: 1090
Re: Test 2 Equations
I'd say review/practice equations that are related to velocity and kinetic energy.
- Tue Oct 23, 2018 7:01 pm
- Forum: Electron Configurations for Multi-Electron Atoms
- Topic: Building- Up Principle
- Replies: 5
- Views: 589
Re: Building- Up Principle
In addition to what everyone else has stated, the half filled d subshell in chromium and completely filled d subshell in copper are more stable configurations because they have comparatively lower energies than [Ar] 3d^5 4s^1 and [Ar] 3d^9 4s^2. I believe this is affected by Hund's rule as well; &qu...
- Thu Oct 11, 2018 1:26 am
- Forum: Photoelectric Effect
- Topic: Work function
- Replies: 7
- Views: 577
Re: Work function
Hi, there are slight differences between the definitions of "work function" and "threshold energy." You can refer to this viewtopic.php?t=15646 for explanations.
- Thu Oct 11, 2018 1:18 am
- Forum: Molarity, Solutions, Dilutions
- Topic: Post Module Assessment
- Replies: 2
- Views: 599
Re: Post Module Assessment
Hi! For this equation, you use the Minitial x Vinitial = Mfinal x Vfinal formula. Essentially, what you're asked to calculate is Mfinal. To do that, just rearrange the formula so it becomes Mfinal = (Minitial x Vinitial) / Vfinal.
- Thu Oct 11, 2018 1:11 am
- Forum: Properties of Electrons
- Topic: atomic spectra
- Replies: 2
- Views: 270
Re: atomic spectra
Exciting an electron (such as that in hydrogen) to a higher energy level means moving it from say n=1 to n=2, given that the frequency of the incoming light that the electron absorbs matches the energy difference between n=1 and n=2. That electron will stay in its "excited state" at n=2 fo...
- Thu Oct 11, 2018 12:42 am
- Forum: Bohr Frequency Condition, H-Atom , Atomic Spectroscopy
- Topic: 6th edition 1.13 part A
- Replies: 1
- Views: 170
Re: 6th edition 1.13 part A
If this question is the one about calculating the wavelength of radiation generated by the electronic transition from n=4 to n=2, I suggest watching the video module entitled Atomic Spectra posted on the chemistry site. You can go to the "Worked Example" part (54:10); professor breaks down...
- Thu Oct 11, 2018 12:26 am
- Forum: Limiting Reactant Calculations
- Topic: Test 1
- Replies: 8
- Views: 1155
Re: Test 1
Hi, I prepared for the test using the Fundamentals exercises in the textbook, and I believe that was enough for me to not fail. So, studying using the homework and modules would be sufficient! I feel like the thing you should prepare for is timing, since you're given 40 minutes for a test that's wor...
- Thu Oct 04, 2018 12:06 pm
- Forum: Balancing Chemical Reactions
- Topic: Extensive v. Intensive Property [ENDORSED]
- Replies: 6
- Views: 2553
Re: Extensive v. Intensive Property [ENDORSED]
Extensive properties depend on size and the amount of matter in the sample that you have. An example of an extensive property is mass. The more you have of something in one sample, the heavier it gets, thus the mass changes. On the other hand, intensive properties depend only on the type of matter i...
- Thu Oct 04, 2018 11:34 am
- Forum: Accuracy, Precision, Mole, Other Definitions
- Topic: Question E29 c.
- Replies: 3
- Views: 326
Re: Question E29 c.
From part a of the question, you should have calculated the amount of CuCl2 4H2O in moles. Then, you can multiply that mole value by the moles of H2O in CuCl2 4H2O, which is 4. Afterwards, you can multiply that product by Avogadro's number to get the number of H2O molecules present. I've attached a ...
- Thu Oct 04, 2018 9:37 am
- Forum: Accuracy, Precision, Mole, Other Definitions
- Topic: problem e15 "sulfide of this metal"
- Replies: 2
- Views: 221
Re: problem e15 "sulfide of this metal"
I believe that your process is correct, Andrea. However, I rounded my answer to 40.08 gmol-1 because the question started with 4 significant figures. Also, since the molar mass is 40.08 gmol-1, the metal would be calcium.
- Thu Oct 04, 2018 9:23 am
- Forum: General Science Questions
- Topic: Moles vs Number of Molecules
- Replies: 5
- Views: 774
Re: Moles vs Number of Molecules
Both are correct in a way, since 1 mol = 6.022x1023 molecules/atoms/formula units/things. But I believe it is more correct to say "6.022x1023 molecules of CH4" instead of just "1 molecule of CH4".

