Search found 54 matches
- Thu Mar 12, 2020 12:07 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: memorizing acids?
- Replies: 4
- Views: 443
Re: memorizing acids?
Definitely memorize the strong acids and strong bases and I think it would be helpful to memorize a few of the most common weak acids (such as acetic acid and formic acid) and weak bases (mostly just ammonia). It helps me to see something on the test that I am familiar with when doing a test for a c...
- Thu Mar 12, 2020 12:02 am
- Forum: Administrative Questions and Class Announcements
- Topic: Format?
- Replies: 11
- Views: 873
Re: Format?
I imagine that it would be similar to the tests that we've taken since most of the time those questions are straightforward. If anything, I would think that the format will be somewhere between the format of the tests and the format of the midterm.
- Wed Mar 11, 2020 11:57 pm
- Forum: Kinetics vs. Thermodynamics Controlling a Reaction
- Topic: Activation Energy
- Replies: 17
- Views: 989
Re: Activation Energy
Activation energy is different from the change in enthalpy. Activation energy is the energy necessary to overcome and break the bonds in the reactants of a reaction that occurs and the reactants only. Enthalpy is the total change in heat, including the amount of heat required to break the bonds of t...
- Wed Mar 11, 2020 11:28 pm
- Forum: General Rate Laws
- Topic: Rate of Formation vs Unique Rate
- Replies: 4
- Views: 460
Re: Rate of Formation vs Unique Rate
Chloe Qiao 4C wrote:I believe they are not the same. Rate of formation depends on the coefficient of the products, but unique rate does not.
How would you determine the unique rate law if these two values are not the same? I'm super lost by how to differentiate between these two values.
- Wed Mar 11, 2020 10:40 pm
- Forum: General Rate Laws
- Topic: Homework 7A.1
- Replies: 4
- Views: 430
Re: Homework 7A.1
It looks like you had the right, but just had the answers flipped around. For part b, it is comparing the rate of NH3 to H2. So when doing this, you would have the balanced number of mols of H2 as the denominator and the number of mols of NH3 on the top. Same idea goes for part C, but now you are lo...
- Thu Mar 05, 2020 2:21 am
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: lnQ vs logQ
- Replies: 5
- Views: 395
Re: lnQ vs logQ
I believe the only difference between the two of these equations is that one uses the natural log and the other uses log base 10. I would just be careful to use the constants we discovered in class when interchanging between these two equations, but other than that they are the same.
- Thu Mar 05, 2020 12:44 am
- Forum: Van't Hoff Equation
- Topic: 5J.15
- Replies: 3
- Views: 435
Re: 5J.15
The data in appendix 2A is measured at STP (25 degrees celsius and 1 atm), so it would work to calculate it for the first portion of the question. However, at 150 degrees celsius you would most likely have to use the Van't Hoff equation. The only way you would be able to use the other equation is if...
- Wed Mar 04, 2020 11:52 pm
- Forum: Van't Hoff Equation
- Topic: Van't Hoff equation
- Replies: 11
- Views: 1283
Re: Van't Hoff equation
Should we be able to derive both versions of the equation or would just know the main derivation be enough?
- Wed Mar 04, 2020 11:20 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: 5G.13
- Replies: 8
- Views: 1740
Re: 5G.13
You are able to first calculate the Q value of the equation. Then, once getting this, you can use the delta G = delta G knot + RTlnQ, but since we do not know the delta G knot, we can replace it with -RTlnK. Thus, the whole equation would be delta G = -RTlnK + RTlnQ. You are able to insert all of t...
- Wed Mar 04, 2020 10:46 pm
- Forum: Work, Gibbs Free Energy, Cell (Redox) Potentials
- Topic: Knowing If K>1 in Redox Reactions
- Replies: 3
- Views: 701
Knowing If K>1 in Redox Reactions
I've been seeing while doing problems to find the standard cell potentials that it also sometimes asks if K > 1 (like 6M.13), but I'm not sure how to go about figuring out this part of the question. Can someone give guidance about how to go about thinking about/solving these kinds of questions? For ...
- Thu Feb 27, 2020 12:24 pm
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: 6N.1
- Replies: 2
- Views: 256
Re: 6N.1
You are correct, I believe that this was a typo made in the book.
- Thu Feb 27, 2020 12:16 pm
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: 6N.1
- Replies: 4
- Views: 419
Re: 6N.1
6N.1 Calculate the equilibrium constants for the following reactions: (b) In3+(aq) + U3+(aq) = In2+(aq) + U4+(aq) I don't know how to do this problem because I can't find the reduction potentials for In 3 to 2+ and the same for Uranium? For part a of this problem, how do you calculate the cell pote...
- Wed Feb 26, 2020 9:09 pm
- Forum: Balancing Redox Reactions
- Topic: Water and H+ Ions in Balanced Redox Reactions
- Replies: 3
- Views: 326
Water and H+ Ions in Balanced Redox Reactions
Why is H2O and H+ used in balanced redox reactions? I've seen it on a couple of problems and I don't understand how to use these in my answers.
- Wed Feb 26, 2020 8:52 pm
- Forum: Balancing Redox Reactions
- Topic: Textbook Question 6K.3
- Replies: 2
- Views: 358
Textbook Question 6K.3
Balance each of the following skeletal equations by using oxidation and reduction half-reactions. All the reactions take place in acidic solution. Identify the oxidizing agent and reducing agent in each reaction. (a) Reaction of thiosulfate ion with chlorine gas The answer key says that the oxidizin...
- Thu Feb 20, 2020 12:28 am
- Forum: Administrative Questions and Class Announcements
- Topic: Number of Chemistry Community Posts
- Replies: 2
- Views: 227
Number of Chemistry Community Posts
Random question, but does anyone know how to check the number of posts you've made on here without making a whole new post or searching through the forums to find the last post you've made?
- Wed Feb 19, 2020 11:53 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Textbook Problem 4J.17
- Replies: 2
- Views: 383
Re: Textbook Problem 4J.17
Assume that Δ H ° and Δ S ° are independent of temperature and use data in Appendix 2A to calculate Δ G ° for each of the following reactions at 80. ° C . Over what temperature range will each reaction be spontaneous under standard conditions? (a) B 2 O 3 (s ) + 6 HF (g ) → 2 BF 3 (g ) + 3 H 2 O (l...
- Wed Feb 19, 2020 3:42 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Textbook Problem 4J.17
- Replies: 2
- Views: 383
Textbook Problem 4J.17
Assume that Δ H ° and Δ S ° are independent of temperature and use data in Appendix 2A to calculate Δ G ° for each of the following reactions at 80. ° C . Over what temperature range will each reaction be spontaneous under standard conditions? (a) B 2 O 3 (s ) + 6 HF (g ) → 2 BF 3 (g ) + 3 H 2 O (l ...
- Wed Feb 19, 2020 2:57 pm
- Forum: Balancing Redox Reactions
- Topic: Oxidation Numbers
- Replies: 7
- Views: 461
Re: Oxidation Numbers
It is common to see them with those oxidation numbers, but there can be exceptions from time to time. I would say to be aware of it and try not to fall into a pattern because it could cause future mistakes!
- Wed Feb 19, 2020 2:53 pm
- Forum: Thermodynamic Systems (Open, Closed, Isolated)
- Topic: Reversible Systems
- Replies: 4
- Views: 457
Re: Reversible Systems
Reversible and irreversible systems have many components to them depending on what you're focusing on, but the main take away is that reversible systems do more than irreversible systems. This is where the differences in components and steps come in; for reversible it is important to note that this ...
- Wed Feb 19, 2020 2:31 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Sign of delta G
- Replies: 9
- Views: 608
Re: Sign of delta G
It would make sense to do this, but I guess it also depends on what information is given within the problem. You would need enough information to calculate the K value if it was not already given and that could take more time than you have.
- Wed Feb 12, 2020 10:47 am
- Forum: Third Law of Thermodynamics (For a Unique Ground State (W=1): S -> 0 as T -> 0) and Calculations Using Boltzmann Equation for Entropy
- Topic: Using heat capacity to determine molar entropy
- Replies: 3
- Views: 267
Re: Using heat capacity to determine molar entropy
I think that the heat capacity is used in the equation \Delta S=\frac{q}{t} , where the heat capacity is used to calculate the value of q using the equation q=mc\Delta T Yes that makes lots of sense thank you! From what I understand now, C=q/mdeltaT , and you can solve for q and use that to find mo...
- Wed Feb 12, 2020 3:13 am
- Forum: Concepts & Calculations Using Second Law of Thermodynamics
- Topic: Boltzmann Formula
- Replies: 3
- Views: 187
Re: Boltzmann Formula
Since it is listed on the learning outcomes outlines and some of the problems assigned include this topic, I would definitely try to make sure to know how to use it and how to solve problems that involve it.
- Wed Feb 12, 2020 3:11 am
- Forum: Concepts & Calculations Using Second Law of Thermodynamics
- Topic: Homework Question 4I.1
- Replies: 2
- Views: 148
Re: Homework Question 4I.1
Yes, you would use that method. The reasoning behind this is that entropy is a state function, so the value is only dependent on its final state rather than the path taken to get there. Because of this, you are able to add together the change in entropy from each step and find the change in entropy...
- Wed Feb 12, 2020 1:24 am
- Forum: Calculating Work of Expansion
- Topic: Reversible and Isobaric
- Replies: 4
- Views: 329
Re: Reversible and Isobaric
I believe that isobaric means constant pressure and in order for that equation to be used, the external pressure has to be held constant. I would be cautious using this though unless it specifically said that the process was irreversible or reversible.
- Wed Feb 12, 2020 1:20 am
- Forum: Calculating Work of Expansion
- Topic: Midterm equation sheet
- Replies: 16
- Views: 732
Re: Midterm equation sheet
Alicia Lin 2F wrote:Will the same exact equation/constant sheet on the course website also be given to us to use on the midterm?
Not necessarily as relevant, but is the equation sheet used on the first test the same as the one that will be given tomorrow?
- Wed Feb 12, 2020 1:17 am
- Forum: Calculating Work of Expansion
- Topic: Positive or negative work
- Replies: 15
- Views: 2178
Re: Positive or negative work
The answer can vary depending on which perspective you are looking at, but when focusing on the system work is positive when work is done on it.
- Wed Feb 05, 2020 9:46 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Hess's Law vs. Standard Enthalpies of Formation
- Replies: 5
- Views: 257
Re: Hess's Law vs. Standard Enthalpies of Formation
I'm sure that the midterm will explicitly say which of these methods to use when solving these kinds of problems if it is not already obvious, much like the textbook problems do. You could probably also utilize the formulas page to try and narrow down the choices if it is unclear.
- Wed Feb 05, 2020 9:14 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: 4E.7
- Replies: 2
- Views: 152
Re: 4E.7
Use the data in Tables 4E.2 and 4E.3 to estimate the reaction enthalpy for b) CH3CHCH2(g) + H2O(g) --> CH3CH(OH)CH3(g) I am really confused on how to determine which bonds are broken/formed. I looked at all the bonds but the solution only used a few. how do we know which ones to use? I also have a ...
- Wed Feb 05, 2020 5:55 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Problem 4D.3
- Replies: 2
- Views: 145
Re: Problem 4D.3
Since you are using a bomb calorimeter, volume is not changing so dU = q + w, where w = PdV. Since volume doesn't change, you can cancel out the w term and change in internal energy is just q. You still have to subtract the heat capacity times the change in pressure to account for the calorimeter. ...
- Wed Feb 05, 2020 3:37 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Question 4C.11
- Replies: 4
- Views: 253
Re: Question 4C.11
When finding the value of q, would you use the specific heat for liquid water or solid water?
- Wed Feb 05, 2020 3:30 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Bomb calorimetry
- Replies: 2
- Views: 157
Re: Bomb calorimetry
I think that because the volume is held constant that points toward using the q equation that is based upon a constant volume.
- Wed Feb 05, 2020 3:26 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: moles or grams in heat capacity equation
- Replies: 3
- Views: 134
Re: moles or grams in heat capacity equation
It depends on the units of the heat capacity equation. I believe that only the equation for specific heat uses grams though, so that is a good starting place for trying to work out or memorize the others.
- Thu Jan 30, 2020 1:46 am
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: 4C.3
- Replies: 4
- Views: 212
Re: 4C.3
Sara Richmond 2K wrote:For part B how do we calculate the change in enthalpy? It is not included in the answer key
The steps I followed were almost exactly the same as the ones shown n example 4C.1 on page 266 in the book. This explains how to find both the final temperature and the change in enthalpy.
- Thu Jan 30, 2020 12:10 am
- Forum: Concepts & Calculations Using Second Law of Thermodynamics
- Topic: 5/2R vs 3/2R
- Replies: 8
- Views: 7906
Re: 5/2R vs 3/2R
KatrinaPho_2I wrote:These values only work if you are dealing with monoatomic ideal gases.
What does you mean by a monoatomic gas?
- Wed Jan 29, 2020 10:35 pm
- Forum: Calculating Work of Expansion
- Topic: 4A.3
- Replies: 8
- Views: 443
4A.3
Air in a bicycle pump is compressed by pushing in the handle. The inner diameter of the pump is 3.0 cm and the pump is depressed 20. cm with a pressure of 2.00 atm. (a) How much work is done in the compression? (b) Is the work positive or negative with respect to the air in the pump? (c) What is the...
- Wed Jan 29, 2020 10:27 pm
- Forum: Calculating Work of Expansion
- Topic: 4A 3
- Replies: 5
- Views: 412
Re: 4A 3
After you calculate the dimension change of the pump, you multiply by the external pressure, which is given as 2 atm. Remember to convert this to pascals, whether that is before or after the calculation. This should give you an answer of 28. Why must you convert the answer to pascals when the units...
- Wed Jan 29, 2020 3:48 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Exothermic and Endothermic
- Replies: 11
- Views: 775
Re: Exothermic and Endothermic
The negative and positive values are in reference to the release and absorption of heat from the perspective of the system itself. Releasing heat produces a negative sign and absorption a positive.
- Wed Jan 29, 2020 3:00 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: internal energy
- Replies: 4
- Views: 205
Re: internal energy
Internal energy is represented as 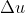 so depending on the question and what values are given, you would use the equation (or its variants)
so depending on the question and what values are given, you would use the equation (or its variants) 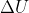 = q + w.
= q + w.
- Wed Jan 29, 2020 2:56 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Reaction Enthalpies
- Replies: 2
- Views: 178
Re: Reaction Enthalpies
I believe they'll be presented on that front page with the formulas and constants, or if not there they will be within the problem itself.
- Thu Jan 23, 2020 12:23 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Converting from Kp to Kc
- Replies: 4
- Views: 168
Re: Converting from Kp to Kc
I can't say if we will have to know how to convert between the two, but I know you should be able to convert from concentration to partial pressure. It was never said explicitly in class that we had to know how to do this, but maybe it is better just to know it in case.
- Thu Jan 23, 2020 1:31 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: ice table
- Replies: 13
- Views: 567
Re: ice table
I usually just put my ice table under the balanced equation and because solids and liquids aren't used to calculate equilibrium, I just write crosses through them as I go along so I don't get confused.
- Thu Jan 23, 2020 1:24 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: 6B. 11
- Replies: 3
- Views: 139
Re: 6B. 11
A student added solid Na2O to a volumetric flask of volume 200.0 mL, which was then filled with water, resulting in 200.0 mL of NaOH solution. Then 5.00 mL of the solution was transferred to another volumetric flask and diluted to 500.0 mL. The pH of the diluted solution is 13.25. (a) What is the m...
- Thu Jan 23, 2020 1:11 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: 6B.9 (a)
- Replies: 3
- Views: 110
Re: 6B.9 (a)
I think sometimes the concentrations can produce negative numbers when calculating pH or pOH because I don't see any other way to find the pH without getting a negative concentration.
- Wed Jan 22, 2020 11:35 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Problem 5I.13
- Replies: 1
- Views: 56
Problem 5I.13
The answer to the question states that the equilibrium of Cl2 is essentially unchanged and then solves the problem by doing 2 * (5.5*10-6). Can someone explain where they found the 5.5*10-6 and why it is multiplied by 2?
- Wed Jan 22, 2020 2:27 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Initial concentrations are the same at equilibrium?
- Replies: 3
- Views: 110
Re: Initial concentrations are the same at equilibrium?
It is possible and commonly seen when doing acid and/or base equilibrium, but I would warn to be careful getting comfortable doing it because there are times when you can't use that rule.
- Thu Jan 16, 2020 11:22 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Quadratic
- Replies: 10
- Views: 301
Re: Quadratic
You would test each value with the equilibrium expression that was found using the ICE table to see if the value is reasonable. Usually one of the values found using the quadratic equation will cause one of the concentrations to be negative, which is not possible.
- Thu Jan 16, 2020 2:10 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: 5J.11
- Replies: 4
- Views: 151
Re: 5J.11
I am assuming that due to the strong bonds between diatomic molecules it would require heat in order to be broken. That would mean that the reaction is endothermic, and therefore an addition of heat would push the reaction towards the products.
- Wed Jan 15, 2020 3:35 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Writing K for heterozygous reactions
- Replies: 5
- Views: 190
Re: Writing K for heterozygous reactions
You wouldn't need to include any of the compounds that are liquid or solid because they are not included in Kc calculations. It is just based on aqueous or gaseous compounds.
- Wed Jan 15, 2020 3:32 pm
- Forum: Ideal Gases
- Topic: "omitting" the units
- Replies: 7
- Views: 660
Re: "omitting" the units
You aren't really "omitting" the units. It is just that since all of the units on the values being used to calculate K[sub]c[sub] are the same, they cancel each other out. It is sort of like when doing conversion factors you put the same units in the denominator or numerator in order to ca...
- Wed Jan 15, 2020 2:41 pm
- Forum: Ideal Gases
- Topic: bars vs atm
- Replies: 8
- Views: 296
Re: bars vs atm
I don't think there is exactly a difference except that they are two different units used to measure pressure. Just make sure that you use the same units that are given in the problem in you calculations and you should be good.
- Sun Jan 12, 2020 10:28 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Units for K
- Replies: 21
- Views: 671
Re: Units for K
Since K is a ratio of values with the same units, they cancel out and are not present in the K value. I would still include them in the K expression to show my work though!
- Sun Jan 12, 2020 10:23 pm
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Inert gas
- Replies: 6
- Views: 179
Re: Inert gas
The addition of inert gases does change the pressure of the system, but the volume remains the same. The number of reactants and products remains the same, therefore there is no reason for a q value to be calculated.
- Sun Jan 12, 2020 9:46 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Reaction Direction
- Replies: 19
- Views: 1040
Re: Reaction Direction
I know that K represents the equilibrium constant, but what does Q represent in this situation? In this situation, Q would represent the state of the reaction at a point where equilibrium has not been reached yet. This is why they are asking about which direction the reaction will occur ("left...
- Wed Jan 08, 2020 8:03 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Finding K without aq
- Replies: 4
- Views: 182
Re: Finding K without aq
Since the K values are based on the species that are aqueous and in your scenario all of the species are solid and/or liquid, the "imaginary coefficient" 1 that is usually ignored due the use of concentrations (or partial pressures) values would be the K value. I believe that Professor Lav...

