Search found 32 matches
- Fri Mar 15, 2019 12:15 pm
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: Concentration Cells
- Replies: 2
- Views: 254
Concentration Cells
Why would reduction take place in the cell with the higher concentration and not the cell with lower concentration?
- Fri Mar 15, 2019 12:12 pm
- Forum: Balancing Redox Reactions
- Topic: Balanced Half Reactions
- Replies: 5
- Views: 696
Balanced Half Reactions
Do the balanced half reactions include the stoichiometric coefficients to make the number of electrons in the oxidation and reduction halves equal, or is a balanced half reaction only referring to the individual half reaction without consideration for the other half? Do we only multiply by coefficie...
- Tue Mar 12, 2019 9:31 pm
- Forum: Reaction Mechanisms, Reaction Profiles
- Topic: Including H2O in Rate Law
- Replies: 1
- Views: 208
Including H2O in Rate Law
When do we know to include H2O in the rate law when given reaction mechanisms that require you to use the pre-equilibrium approach (for example, problem 15.101 in the sixth edition textbook)? Will a problem generally indicate when water is a solvent and when it isn't? Thank you in advance!
- Sun Mar 10, 2019 10:36 pm
- Forum: First Order Reactions
- Topic: Pseudo rate laws
- Replies: 2
- Views: 296
Pseudo rate laws
Can someone explain what a pseudo rate law is, and in what context we would use a pseudo rate law? Also, practically speaking, why is it important for us to use and understand pseudo rate laws? Thank you in advance!
- Sun Mar 10, 2019 3:09 pm
- Forum: Reaction Mechanisms, Reaction Profiles
- Topic: Microscopic Reversibility
- Replies: 2
- Views: 342
Microscopic Reversibility
Dr. Lavelle mentioned that microscopic reversibility is assumed/applied when K>>1 and k is much larger than k'. Can someone please explain what microscopic reversibility is and how it applies to forward and reverse reactions? Thank you in advance!
- Sat Mar 09, 2019 9:06 pm
- Forum: First Order Reactions
- Topic: 15.27 6th edition
- Replies: 1
- Views: 250
15.27 6th edition
Can someone explain what relationship or equation the solution manual used to solve for time in parts a and b in question 15.27? I did the entire problem by solving for the rate constant first and then using the first order integrated rate law, as the solution manual did for parts c and d, but I'm c...
- Sun Mar 03, 2019 4:01 pm
- Forum: General Rate Laws
- Topic: Initial Reaction Rates
- Replies: 2
- Views: 302
Initial Reaction Rates
Why is it easier to study initial reaction rates rather than rates later in the reaction?
- Sun Mar 03, 2019 3:58 pm
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: Low voltage in concentration cells
- Replies: 2
- Views: 361
Low voltage in concentration cells
Why is  and why is E typically low voltage for a concentration cell?
and why is E typically low voltage for a concentration cell?
- Mon Feb 25, 2019 12:19 am
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Conductors and Cell Diagrams
- Replies: 1
- Views: 202
Conductors and Cell Diagrams
How do you know when to put an inert conductor into a cell diagram?
- Sun Feb 24, 2019 5:34 pm
- Forum: Balancing Redox Reactions
- Topic: Order to balance
- Replies: 6
- Views: 579
Order to balance
When balancing half reactions, should we balance the electrons or the moles first? Also, we do we multiply the number of electrons by a stoichiometric coefficient?
- Sun Feb 24, 2019 5:15 pm
- Forum: Balancing Redox Reactions
- Topic: Balancing redox reactions in basic solutions
- Replies: 2
- Views: 400
Balancing redox reactions in basic solutions
Is there a way to tell which side of the reaction the OH- and the H2O should go on or is it just a matter of trying both until the equation is balanced?
- Sat Feb 23, 2019 11:56 pm
- Forum: Balancing Redox Reactions
- Topic: Rules for oxidation numbers
- Replies: 2
- Views: 424
Rules for oxidation numbers
What are the rules that we should know for assigning oxidation numbers? Thanks!
- Sat Feb 16, 2019 9:07 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: State Function
- Replies: 2
- Views: 336
State Function
Why is change in internal energy a state function when it is comprised of things that are not state functions?
- Sat Feb 16, 2019 9:06 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Equilibrium Constant and Gibbs Free Energy
- Replies: 3
- Views: 423
Equilibrium Constant and Gibbs Free Energy
I'm a little confused as to how the equation 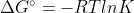 is derived.
is derived.
Where does the equilibrium constant come from in this equation? Thanks!
Where does the equilibrium constant come from in this equation? Thanks!
- Sat Feb 16, 2019 12:26 am
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Max work
- Replies: 1
- Views: 230
Max work
Why is max work equal to the change in Gibbs free energy and what conditions must be met in order for this relationship to exist?
- Sun Feb 10, 2019 9:43 pm
- Forum: Third Law of Thermodynamics (For a Unique Ground State (W=1): S -> 0 as T -> 0) and Calculations Using Boltzmann Equation for Entropy
- Topic: Entropy of the Universe
- Replies: 1
- Views: 223
Entropy of the Universe
Why is the entropy of the universe greater than zero for a spontaneous reaction?
- Sun Feb 10, 2019 12:31 am
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: Delta U
- Replies: 8
- Views: 885
Delta U
When is 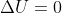 ?
?
- Thu Feb 07, 2019 9:57 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Limiting Reagents
- Replies: 5
- Views: 654
Limiting Reagents
In what situations would you need to calculate the limiting reagent of a chemical reaction?
- Sun Feb 03, 2019 2:18 pm
- Forum: Calculating Work of Expansion
- Topic: Units for pressure
- Replies: 10
- Views: 789
Units for pressure
When calculating work, does it matter what unit of pressure is being used? Or should we convert whatever unit of pressure we are given to atmospheres?
- Fri Feb 01, 2019 11:57 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: Internal Energy
- Replies: 3
- Views: 509
Internal Energy
The textbook states that the internal energy of a monatomic ideal gas at a constant temperature is 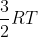 . How is this expression derived?
. How is this expression derived?
- Mon Jan 28, 2019 9:02 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Cp and Cv
- Replies: 3
- Views: 369
Cp and Cv
The textbook and homework questions reference the constant R often when calculating heat given off by a system -- for example, the molar heat capacity of a monatomic ideal gas at constant pressure is C_{P,m}=\frac{5}{2}R Is this R supposed to by the ideal gas constant and are we supposed to use the ...
- Sun Jan 27, 2019 11:01 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Heat and Enthalpy
- Replies: 2
- Views: 208
Heat and Enthalpy
How can we distinguish between heat and enthalpy, conceptually?
- Fri Jan 25, 2019 7:57 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Standard enthalpy of formation
- Replies: 2
- Views: 207
Standard enthalpy of formation
I'm a little confused what standard enthalpy of formation is. Can someone please explain it and its relationship to standard reaction enthalpy?
- Mon Jan 21, 2019 3:31 pm
- Forum: Calculating pH or pOH for Strong & Weak Acids & Bases
- Topic: 6th edition 12.81
- Replies: 1
- Views: 218
6th edition 12.81
The question says to calculate pH and ignore the second second deprotonation only when the approximation is justified. What approximation should I be using in order to make this judgement, and why does the approximation allow me to ignore the second deprotonation?
- Fri Jan 18, 2019 10:39 pm
- Forum: Calculating pH or pOH for Strong & Weak Acids & Bases
- Topic: 6th edition 12.45
- Replies: 1
- Views: 202
6th edition 12.45
I understand how to compare the strengths of bases based on the pka value of their conjugate bases, but the solution manual gives a further explanation of, "Although we should not draw conclusions from such a small data set, we might suggest the possibility that 1) arylamines < ammonia < alkyla...
- Thu Jan 17, 2019 5:42 pm
- Forum: Acidity & Basicity Constants and The Conjugate Seesaw
- Topic: Strong acids and bases
- Replies: 2
- Views: 337
Strong acids and bases
What are the strong acids and bases that we should memorize?
- Mon Jan 14, 2019 10:52 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: 6th edition 11.89
- Replies: 1
- Views: 162
6th edition 11.89
The balanced equation for this reaction is supposed to be 2A <--> B+ 2C. Can someone explain how we get the stoichiometric coefficients by looking at the graph provided? Thank you!
- Thu Jan 10, 2019 9:29 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Definition of equilibrium
- Replies: 4
- Views: 210
Re: Definition of equilibrium
A reaction has reached equilibrium when the concentration of reactants and products no longer changes. Reactions are still occurring, but the rate at which reactants become products is equal to the rate at which products become reactants. For example in the reaction A\leftrightharpoons B , equilibri...
- Thu Jan 10, 2019 9:11 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Help with question 11.43 6th edition [ENDORSED]
- Replies: 2
- Views: 251
Re: Help with question 11.43 6th edition [ENDORSED]
Oh that makes sense. Thank you!
- Thu Jan 10, 2019 8:50 pm
- Forum: Ideal Gases
- Topic: Pv=nRT [ENDORSED]
- Replies: 12
- Views: 791
Re: Pv=nRT [ENDORSED]
Yes, you're right, ideal gas law uses temperature in Kelvin
- Wed Jan 09, 2019 10:55 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Help with question 11.43 6th edition [ENDORSED]
- Replies: 2
- Views: 251
Help with question 11.43 6th edition [ENDORSED]
When determining the equilibrium relation, I understand where the terms x^2 and 2x come from, but I don't quite understand why we subtract 2x from the initial partial pressure of NO to create the expression of 1-2x. Can someone please explain this? Thank you!
- Tue Jan 08, 2019 9:36 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: 11.7
- Replies: 5
- Views: 466
Re: 11.7
For part b, it's asking for the percentage of molecules that have decomposed, so you want to use a ratio of the number of diatomic molecules in flask three minus the number in flask one, to the number of diatomic molecules you began with in flask one. Taking the difference between the number of diat...

