Search found 31 matches
- Tue Mar 12, 2019 3:38 pm
- Forum: Method of Initial Rates (To Determine n and k)
- Topic: Unique rate?
- Replies: 7
- Views: 652
Re: Unique rate?
The unique rate depends on the stoichiometric coefficients of the products or reactants, which differs from k, the rate constant.
- Tue Mar 12, 2019 3:36 pm
- Forum: Reaction Mechanisms, Reaction Profiles
- Topic: Slow Step
- Replies: 6
- Views: 519
Re: Slow Step
On finals in previous years there has been questions where it is not given, I've heard, but because the slow step determines the overall rate law we should be able to figure it out with the given information if it is not given.
- Tue Mar 12, 2019 3:35 pm
- Forum: First Order Reactions
- Topic: Half-Life
- Replies: 7
- Views: 792
Re: Half-Life
In addition, half life is often used for carbon dating of samples, for example fossils, to determine from the amount of decay the sample has undergone. There is no normal life because that would imply that it is the time it takes for the entire sample to decay.
- Sun Mar 03, 2019 4:21 pm
- Forum: Balancing Redox Reactions
- Topic: Acidic Solutions
- Replies: 7
- Views: 869
Re: Acidic Solutions
Balance the equation first by adding H2O to the side that lacks oxygen, then add H+ ions to the other side to balance it overall.
- Sun Mar 03, 2019 4:20 pm
- Forum: Work, Gibbs Free Energy, Cell (Redox) Potentials
- Topic: Pt inert electrode
- Replies: 9
- Views: 1814
Re: Pt inert electrode
Pt is added to a reaction that does not have a conducting solid, and is added on whichever side needs it, not on both sides.
- Sun Mar 03, 2019 4:18 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Order of Cell Diagrams
- Replies: 13
- Views: 1592
Re: Order of Cell Diagrams
From the left, the order should be Solids|Gas, Liquid|Aqueous solutions||Aqueous solutions|Liquid, Gas|Solids
- Mon Feb 25, 2019 6:18 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Test 2
- Replies: 4
- Views: 515
Re: Test 2
The second test covers all material up to and including what was discussed in class on Friday 2/22
- Sun Feb 24, 2019 5:26 pm
- Forum: Balancing Redox Reactions
- Topic: Balancing redox reactions in basic solutions
- Replies: 2
- Views: 400
Re: Balancing redox reactions in basic solutions
In acidic solution, balance O by using H2O and then balance H by using H+. In basic solution, balance O by using H2O; then balance H by adding H2O to the side of each half- reaction that needs H and adding OH- to the other side.
- Sun Feb 24, 2019 1:53 am
- Forum: Balancing Redox Reactions
- Topic: Rules for oxidation numbers
- Replies: 2
- Views: 424
Re: Rules for oxidation numbers
1) The oxidation number for an atom in its elemental form is always zero. 2) The oxidation number of a monoatomic ion = charge of the monatomic ion. 3) The sum of all oxidation numbers in a neutral compound is zero. The sum of all oxidation numbers in a polyatomic ion is equal to the charge on the i...
- Sat Feb 16, 2019 9:59 pm
- Forum: Ideal Gases
- Topic: Q and K
- Replies: 13
- Views: 1330
Re: Q and K
Q doesn't mean anything until it is compared to K. The comparison helps to determine which direction the reaction will proceed given time.
- Sat Feb 16, 2019 9:57 pm
- Forum: Third Law of Thermodynamics (For a Unique Ground State (W=1): S -> 0 as T -> 0) and Calculations Using Boltzmann Equation for Entropy
- Topic: How to calculate W
- Replies: 9
- Views: 1470
Re: How to calculate W
W is calculated by ^{(number of molecules)})
and then is plugged into the equation
to find the statistical/residual entropy
and then is plugged into the equation
to find the statistical/residual entropy
- Sat Feb 16, 2019 9:55 pm
- Forum: Entropy Changes Due to Changes in Volume and Temperature
- Topic: delta S
- Replies: 7
- Views: 768
Re: delta S
- Sat Feb 16, 2019 9:54 pm
- Forum: Concepts & Calculations Using Second Law of Thermodynamics
- Topic: ΔS=q/t
- Replies: 8
- Views: 933
Re: ΔS=q/t
when pressure and temperature are constant, 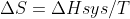
- Sat Feb 16, 2019 9:51 pm
- Forum: Non-Equilibrium Conditions & The Reaction Quotient
- Topic: K constant
- Replies: 5
- Views: 961
Re: K constant
The brackets [Kc] is for calculating equilibrium concentrations (done in M) where (Kp) is for calculation equilibrium pressure (done in atm or barr)
- Sat Feb 16, 2019 9:49 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Midterm
- Replies: 12
- Views: 2161
Re: Midterm
I would say to just do as many homework problems as you can to familiarize yourself with the way these concepts take hold in actual problems, although it is also important to understand the ideas behind the problems as well. I didn't do enough homework problems and it totally screwed me over on the ...
- Sat Feb 16, 2019 9:45 pm
- Forum: Ideal Gases
- Topic: pH and pOH
- Replies: 18
- Views: 1971
Re: pH and pOH
The problem should specify, but if not it is usually safe to assume you should calculate for what is given, ie if the reaction forms H+ ions you should solve for pH and if OH- ions are formed solve for pOH.
- Sat Feb 16, 2019 9:44 pm
- Forum: Entropy Changes Due to Changes in Volume and Temperature
- Topic: Changes in Pressure
- Replies: 5
- Views: 585
Re: Changes in Pressure
because of the ideal gas law, pressure and volume have an inverse relationship and thus when volume increases, pressure decreases.
- Sat Feb 16, 2019 9:42 pm
- Forum: Third Law of Thermodynamics (For a Unique Ground State (W=1): S -> 0 as T -> 0) and Calculations Using Boltzmann Equation for Entropy
- Topic: Explaining Boltzmann's Equation
- Replies: 6
- Views: 1401
Re: Explaining Boltzmann's Equation
Boltzmann's equation solves for statistical or residual entropy, and is based on the number of microstates and number of molecules in a system.
- Sat Feb 16, 2019 9:40 pm
- Forum: Thermodynamic Systems (Open, Closed, Isolated)
- Topic: Constant Pressure Calorimeter
- Replies: 5
- Views: 967
Re: Constant Pressure Calorimeter
Calorimeters are considered isolated systems in problems, although it is very difficult to create a true isolated system in real life calorimeters are pretty close and are considered isolated.
- Sat Feb 16, 2019 9:38 pm
- Forum: Entropy Changes Due to Changes in Volume and Temperature
- Topic: Calculating Delta S with a change in temperature
- Replies: 5
- Views: 592
Re: Calculating Delta S with a change in temperature
It is usually Cp unless the volume is changing
- Sat Feb 16, 2019 9:33 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Equilibrium Constant and Gibbs Free Energy
- Replies: 3
- Views: 423
Re: Equilibrium Constant and Gibbs Free Energy
If Gr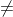 Gp, then the rxn is not at equilibrium and:
Gp, then the rxn is not at equilibrium and:
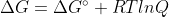
At Equilibrium, = 0
= 0
Resulting in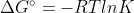
At Equilibrium,
Resulting in
- Sat Feb 16, 2019 9:12 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: State Function
- Replies: 2
- Views: 336
Re: State Function
The change in internal energy during a process depends only upon the initial state and final state while work and heat depends on upon the path followed. Thus, internal energy is a state function and work and heat are not.
- Sat Feb 16, 2019 9:06 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: 3/2R vs 5/2R
- Replies: 8
- Views: 2085
Re: 3/2R vs 5/2R
3/2 R is used for a monoatomic ideal gas at constant volume where 5/2 R is used is used for a monoatomic gas at constant pressure, or for a linear molecule at constant volume.
- Mon Jan 28, 2019 4:12 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Delta H
- Replies: 4
- Views: 502
Re: Delta H
Delta T indicates a change in temperature where delta H indicates a change in energy, or enthalpy
- Mon Jan 28, 2019 4:10 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Water specific heat capacity
- Replies: 2
- Views: 266
Re: Water specific heat capacity
Water has such a high specific heat capacity because of multiple hydrogen bonds between water molecules it takes a lot amount of heat energy to cause those molecules to move faster and raise the temperature of the water.
- Mon Jan 28, 2019 4:08 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Exothermic vs. Endothermic
- Replies: 10
- Views: 3196
Re: Exothermic vs. Endothermic
If the reaction gives a release of heat (-delta H), the reaction is exothermic. If the reaction requires heat (+delta H), the reaction is endothermic.
- Mon Jan 21, 2019 8:27 pm
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: 5% rule
- Replies: 12
- Views: 21806
Re: 5% rule
If the percent protonation is <5%, then the approximation is valid and allows for finding x without using the quadratic formula
- Mon Jan 21, 2019 8:25 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Kp vs Kc
- Replies: 7
- Views: 770
Re: Kp vs Kc
Kp refers to pressure where Kc refers to concentration
- Mon Jan 21, 2019 8:24 pm
- Forum: Ideal Gases
- Topic: ICE table
- Replies: 11
- Views: 1330
Re: ICE table
ICE tables can be used for both pressure and molarity.
- Thu Jan 10, 2019 10:37 pm
- Forum: General Science Questions
- Topic: Chem 20A to Chem 14B
- Replies: 3
- Views: 807
Chem 20A to Chem 14B
Hi all, I took Chem 20A last quarter and my advisor said that there should be no issue for me in taking Chem 14B this quarter. However, I've noticed that the material differs drastically from the 20 series and I was wondering, realistically, how transferrable is the knowledge from the 20 series to t...
- Thu Jan 10, 2019 10:32 pm
- Forum: Student Social/Study Group
- Topic: Post All Chemistry Jokes Here
- Replies: 9651
- Views: 3705752
Re: Post All Chemistry Jokes Here
Two scientists sit down at a restaurant
The first scientist: I'll have H2O
The second scientist: I'll have H20, also.
The second scientist’s arch nemesis disguised as a waiter: [under breath] so close....
The first scientist: I'll have H2O
The second scientist: I'll have H20, also.
The second scientist’s arch nemesis disguised as a waiter: [under breath] so close....

