Search found 50 matches
- Thu Mar 12, 2020 4:59 pm
- Forum: Method of Initial Rates (To Determine n and k)
- Topic: 7A.11) 700K?
- Replies: 6
- Views: 498
Re: 7A.11) 700K?
Yes, I think you can ignore it since temperature isn't part of the equation for reaction rate.
- Thu Mar 12, 2020 4:50 pm
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: 6.43 questions
- Replies: 2
- Views: 226
Re: 6.43 questions
6.43
a) applies to Ecell only
b) both are temperature dependent (look at the equations for both)
c) neither --> both are dependent on n, so they won't change if you multiply by a constant
d) Ecell naught only because Ecell=0 at equilibrium
e) Ecell only
a) applies to Ecell only
b) both are temperature dependent (look at the equations for both)
c) neither --> both are dependent on n, so they won't change if you multiply by a constant
d) Ecell naught only because Ecell=0 at equilibrium
e) Ecell only
- Thu Mar 12, 2020 4:45 pm
- Forum: Work, Gibbs Free Energy, Cell (Redox) Potentials
- Topic: n in NFE
- Replies: 64
- Views: 3867
Re: n in NFE
n is the number of electrons being transferred (after balancing the half-reactions)
- Thu Mar 12, 2020 4:39 pm
- Forum: Reaction Mechanisms, Reaction Profiles
- Topic: reaction profiles
- Replies: 3
- Views: 273
Re: reaction profiles
Is this the same as the graphs we use to determine reaction rates?
- Thu Mar 12, 2020 4:33 pm
- Forum: Student Social/Study Group
- Topic: Review Session???
- Replies: 3
- Views: 452
Re: Review Session???
I'm pretty sure all the review sessions were cancelled and there's no online ones in place... but I know some students were planning on doing their own review session going through the endgame problems
- Fri Mar 06, 2020 9:28 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Reducing/Oxidizing agents
- Replies: 5
- Views: 403
Re: Reducing/Oxidizing agents
A reducing agent just means the species that is oxidized, AKA the species that's giving up electrons (since those electrons REDUCE the other species, this is called the reducing agent). An oxidizing agent is the opposite--it's the species that is reduced, since it's gaining electrons, it must take t...
- Fri Mar 06, 2020 9:14 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Signs of Enaught
- Replies: 7
- Views: 609
Re: Signs of Enaught
What does electrolytic mean?
- Fri Mar 06, 2020 9:00 pm
- Forum: Work, Gibbs Free Energy, Cell (Redox) Potentials
- Topic: pH and Oxidizing Strength
- Replies: 3
- Views: 303
Re: pH and Oxidizing Strength
Also, how do you know if a metal/other compound is a strong reducing/oxidizing agent?
- Fri Mar 06, 2020 8:57 pm
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: Cell potentials and concentration
- Replies: 3
- Views: 282
Cell potentials and concentration
How does changing the concentration in a concentration cell affect the cell potential?
- Fri Mar 06, 2020 8:52 pm
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: Nernst Equation for Concentration Cells
- Replies: 6
- Views: 406
Re: Nernst Equation for Concentration Cells
How do you know what n is when the half reactions have different numbers of electrons being transferred? Would you balance them and use that number?
- Sun Mar 01, 2020 11:40 pm
- Forum: Ideal Gases
- Topic: Conjugate acid and bases
- Replies: 7
- Views: 563
Re: Conjugate acid and bases
ursulavictorino1K wrote:Is a base a proton acceptor or donor?
A base is a proton acceptor!
- Sun Mar 01, 2020 11:34 pm
- Forum: Balancing Redox Reactions
- Topic: half rxn or ionization
- Replies: 3
- Views: 280
Re: half rxn or ionization
I think you just have to know which molecules actually form ionic bonds, and assume all the others are just conceptual.
- Sun Mar 01, 2020 11:32 pm
- Forum: Balancing Redox Reactions
- Topic: Cell Diagrams
- Replies: 2
- Views: 232
Re: Cell Diagrams
I also think this is correct! It makes sense if you kind of match up each phase with the galvanic cell diagrams we drew in class.
- Sun Mar 01, 2020 11:29 pm
- Forum: General Rate Laws
- Topic: Preferred way to write reaction rate
- Replies: 5
- Views: 404
Re: Preferred way to write reaction rate
I think you can do it either way, it's just easier to have the specific product/reactant that you're focused on, without a fraction in front.
- Sun Mar 01, 2020 11:23 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Electromotive force
- Replies: 3
- Views: 243
Re: Electromotive force
Also, what is the significance of electromotive force? I don't remember what Dr. Lavelle said about it in lecture.
- Sun Feb 23, 2020 8:29 pm
- Forum: Van't Hoff Equation
- Topic: deltaS/R
- Replies: 7
- Views: 496
Re: deltaS/R
I'm not sure what you mean, could you give an example of what you're referring to?
- Sun Feb 23, 2020 8:29 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Cell Diagram
- Replies: 4
- Views: 262
Re: Cell Diagram
Yes, like everyone has said, I think cell diagrams are just a way for you to visualize what's happening during a redox reaction, as well as see what materials are used and which reactants are being reduced/oxidized, etc.
- Sun Feb 23, 2020 8:22 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: 6L. 3
- Replies: 4
- Views: 322
Re: 6L. 3
Also how do you know where everything goes in a cell diagram?
- Sun Feb 23, 2020 8:17 pm
- Forum: Balancing Redox Reactions
- Topic: Oxidation Numbers/States
- Replies: 8
- Views: 545
Re: Oxidation Numbers/States
How do you use the oxidation numbers/states to get the half reactions? Also do we need to memorize the oxidation numbers for common molecules/elements?
- Sun Feb 23, 2020 8:08 pm
- Forum: Balancing Redox Reactions
- Topic: Steps
- Replies: 7
- Views: 430
Re: Steps
To balance redox reactions, you want to balance the electrons transferred in the reduction reaction and the oxidation reaction. To do so, you want to separate the whole reaction into two half reactions: the reduction reaction (gaining electrons) and the oxidation reaction (losing reaction) by writi...
- Sat Feb 15, 2020 9:13 am
- Forum: Entropy Changes Due to Changes in Volume and Temperature
- Topic: Rules for constant pressure
- Replies: 4
- Views: 277
Rules for constant pressure
What equations/assumptions do you use when it tells you there is constant pressure? What about constant volume?
- Sat Feb 15, 2020 9:08 am
- Forum: General Science Questions
- Topic: Converting Liters to m^3 ?
- Replies: 2
- Views: 248
Converting Liters to m^3 ?
Hello, this may be a dumb question but I don't get how to convert liters to cubic meters. I know on the formula sheet it says 1 L=1 dm^3 but I have absolutely no clue what a dm is. Thanks!
- Sat Feb 15, 2020 9:02 am
- Forum: Calculating the pH of Salt Solutions
- Topic: How do you know if something is a salt solution?
- Replies: 6
- Views: 738
How do you know if something is a salt solution?
Whenever I encounter a salt solution/buffer problem, I immediately get confused. Can someone explain what exactly a salt solution is, and how to go about finding the pH? I don't really understand the context of these questions so I think that's why I'm confused. It would be awesome if someone could ...
- Sat Feb 15, 2020 8:56 am
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Equilibrium
- Replies: 5
- Views: 264
Re: Equilibrium
I know delta G is for sure 0 at equilibrium, since there's no change in the free energy, but I'm not sure about delta H or delta S. I don't think they would be at zero, since the reaction is still occurring (because equilibrium is dynamic).
- Tue Feb 11, 2020 11:13 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: 4D.15 Sum of enthalpy in equation is positive, but answer is negative
- Replies: 1
- Views: 165
Re: 4D.15 Sum of enthalpy in equation is positive, but answer is negative
I had the exact same question! The first time I did it, I got +312kJ by subtracting the enthalpies of formation of reactants (C2H2 and H2) from the enthalpy of formation of product (C2H6). But in the solution, it looks like they just added all the enthalpies of formation, but flipped the sign of the...
- Tue Feb 11, 2020 12:04 am
- Forum: Third Law of Thermodynamics (For a Unique Ground State (W=1): S -> 0 as T -> 0) and Calculations Using Boltzmann Equation for Entropy
- Topic: Using Cv and Cp
- Replies: 4
- Views: 257
Re: Using Cv and Cp
How do we know when to use Cv, and when to use Cp?
- Mon Feb 10, 2020 11:18 pm
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: pKb to pOH
- Replies: 2
- Views: 78
Re: pKb to pOH
I'm not sure you'd be able to directly find pOH if you were only given pKb. Pretty sure you would also need the initial concentration of reactant/product or some other values to plug into your ICE table, for which you would use pKb to find the pOH.
- Thu Feb 06, 2020 12:00 pm
- Forum: Thermodynamic Systems (Open, Closed, Isolated)
- Topic: Specific Heat Capacity
- Replies: 3
- Views: 267
Re: Specific Heat Capacity
Since Q denotes the amount of heat transferred to a system, a negative q value would mean that the system is releasing heat, and a positive q would mean that heat is going into the system. But usually we use enthalpy (∆H) to determine whether a reaction is endothermic or exothermic. Enthalpy is simp...
- Thu Feb 06, 2020 11:53 am
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: Isothermal Process Slow Expansion
- Replies: 2
- Views: 190
Re: Isothermal Process Slow Expansion
I think it's because if a process is isothermal, it means the temperature isn't changing, so there's essentially no transfer of heat, so q=0. Then, since q=w, as you stated, w also equals 0. Internal energy = q+w, so 0+0=0.
- Fri Jan 31, 2020 10:10 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Textbook section 4B.3: A Molecular Interlude
- Replies: 1
- Views: 61
Re: Textbook section 4B.3: A Molecular Interlude
I saw that too. I don't think we have gone over those things in class, and I'm not sure we will. But maybe we'll come back to it? Not sure, I'd skip that reading for now but it probably wouldn't hurt to read it.
- Fri Jan 31, 2020 10:08 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Work done by expansion
- Replies: 8
- Views: 188
Re: Work done by expansion
I don't believe he's covered this yet, but we do have the general equation 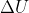 =q+w.
=q+w.
- Fri Jan 31, 2020 10:07 pm
- Forum: Thermodynamic Systems (Open, Closed, Isolated)
- Topic: Isolated vs Closed [ENDORSED]
- Replies: 34
- Views: 4324
Re: Isolated vs Closed [ENDORSED]
In a closed system, heat can still be transferred, but in an isolated system, neither heat nor matter can be transferred.
- Fri Jan 31, 2020 10:05 pm
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Equilibrium shift by pressure
- Replies: 7
- Views: 269
Re: Equilibrium shift by pressure
Yes, the equilibrium shift only occurs if the pressure changes due to changes in volume. For example, if a gas is compressed, or the volume is decreased, then the pressure increases and equilibrium shifts to the direction with less moles.
- Fri Jan 31, 2020 10:03 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Test 1 # 4
- Replies: 10
- Views: 386
Re: Test 1 # 4
This one tricked me too! You have to use the ideal gas law, so PV=nRT and just plug everything in. But make sure to convert everything to the right units.
- Tue Jan 28, 2020 10:17 pm
- Forum: Calculating pH or pOH for Strong & Weak Acids & Bases
- Topic: 6D.9 - Calculating pH and Ka for benzoic acid
- Replies: 1
- Views: 760
6D.9 - Calculating pH and Ka for benzoic acid
This problem says: The percentage deprotonation of benzoic acid in a 0.110M solution is 2.4%. What is the pH of the solution and the Ka of benzoic acid? I understand how to use the percentage deprotonation to find the pH, but I am confused on how to find Ka. In the solutions manual, I have the same ...
- Thu Jan 23, 2020 12:24 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: 6B.9 (a)
- Replies: 3
- Views: 110
Re: 6B.9 (a)
I was also really confused on how to get the answer to parts (i) and (ii) of this question. How come you can't use the given value of [H30+]=1.5 mol.L-1 and then substitute into [H30+][OH-]=10^-14 to find [OH-]? I tried doing that, but I kept getting the wrong answer.
- Thu Jan 23, 2020 12:19 am
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Changes in pressure
- Replies: 5
- Views: 154
Changes in pressure
Why is it that when a system's pressure is increased by decreasing the volume, the reaction will shift toward the side with less moles, but when a system's pressure is increased by adding an inert gas, nothing happens?
- Thu Jan 23, 2020 12:15 am
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Le chatelier and Temperature
- Replies: 9
- Views: 313
Re: Le chatelier and Temperature
if delta h is positive for a reaction (aka endothermic, or requires heat), and you increase the temperature, then the reaction will favor the formation of products since the heat is used to make products. As a result, K will increase for endothermic reactions when the temperature is increased. On th...
- Thu Jan 23, 2020 12:08 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: 5% rule
- Replies: 13
- Views: 641
Re: 5% rule
I think you use it anytime you make an approximation, or assume that x is so small that it's essentially equal to 0.
- Thu Jan 23, 2020 12:04 am
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Buffers
- Replies: 3
- Views: 122
Re: Buffers
I don't believe that buffers will be on the test, but you should definitely know how to find the pH of weak acids/bases.
- Thu Jan 23, 2020 12:03 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Hw I.15 ICE table
- Replies: 1
- Views: 142
Re: Hw I.15 ICE table
I'm pretty sure you would use the same process as if there were reactants used in the equation. Still make an ICE table, putting in the information that is known (initial concentration of NH3 is given), and then assume that both products increase by x, so you would have [NH3]=0.200+x and [H2S]=x as ...
- Wed Jan 22, 2020 11:48 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: 6D.3
- Replies: 1
- Views: 62
Re: 6D.3
Yes, so you would do the same process as usual to find the equilibrium concentration for HClO2. You can create an ICE table, fill in the known values, which in this case, you know the initial concentration of HClO2. Then, you can call the change in concentration for each molecule in the reaction &qu...
- Wed Jan 22, 2020 9:35 am
- Forum: Acidity & Basicity Constants and The Conjugate Seesaw
- Topic: 6D.15 part b
- Replies: 3
- Views: 274
Re: 6D.15 part b
The Cl is not included in the equation because it's not involved in the reaction, so it would just cancel out anyway.
- Wed Jan 22, 2020 1:51 am
- Forum: Acidity & Basicity Constants and The Conjugate Seesaw
- Topic: Homework 6C.5
- Replies: 2
- Views: 561
Re: Homework 6C.5
The conjugate base for HCOOH would be HCOO-, and since the pKa is 3.75, you can subtract that from 14 (pKa+pKb=pKw=14), and get 10.25 for the pKb.
- Wed Jan 22, 2020 12:26 am
- Forum: Non-Equilibrium Conditions & The Reaction Quotient
- Topic: Calculating K
- Replies: 15
- Views: 773
Re: Calculating K
You can only calculate K for equations that are at equilibrium, which is usually indicated in the problem. If the equation is not balanced, then balance it and then find K (assuming the reaction is at equilibrium). Conversely, Q is calculated when the reaction is NOT at equilibrium, and is simply th...
- Sun Jan 12, 2020 9:04 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Solids and Liquids
- Replies: 6
- Views: 153
Re: Solids and Liquids
Solids and liquids typically don't change concentrations at all during reactions, so they are not included in the equilibrium constant.
- Sun Jan 12, 2020 8:52 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: K and Q
- Replies: 10
- Views: 314
Re: K and Q
Q is the reaction quotient, whereas K is the equilibrium constant. While they are both ratios of product to reactant, K represents the ratio when the reaction is at equilibrium, or when the forward rate of the reaction is equal to the reverse rate (products are being formed at the same rate as react...
- Sun Jan 12, 2020 8:36 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Kp vs Kc usage
- Replies: 5
- Views: 192
Re: Kp vs Kc usage
I think if the problem is dealing with gases, or it gives partial pressures, then you should use Kp, and if it's talking about concentrations then use Kc.
- Sun Jan 12, 2020 7:29 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: K units
- Replies: 3
- Views: 112
Re: K units
Technically, the equilibrium constant (K) does not have units because it uses the activities of the reactants/products as its terms, rather than concentrations or partial pressures.
- Sun Jan 12, 2020 6:02 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Homework 5G.1 Help
- Replies: 4
- Views: 353
Re: Homework 5G.1 Help
Make sure you're reading the question very closely. Part D is comparing the initial concentrations of reactant (what you start with) and equilibrium concentrations of product (what you end up with once the reaction reaches equilibrium). It says that if you START with higher concentrations of reactan...

