Search found 57 matches
- Thu Mar 12, 2020 10:36 am
- Forum: Second Order Reactions
- Topic: Finding out order
- Replies: 22
- Views: 1068
Re: Finding out order
For order (m + n), the rate constant has units of mol1−(m+n)·L(m+n)−1·s−1 For order zero, the rate constant has units of mol·L−1·s−1 (or M·s−1) For order one, the rate constant has units of s−1 For order two, the rate constant has units of L·mol−1·s−1 (or M−1·s−1) And for order three, the rate const...
- Thu Mar 12, 2020 10:33 am
- Forum: First Order Reactions
- Topic: Units for t
- Replies: 30
- Views: 1365
Re: Units for t
It easiest if you change the t value to the time value of your rate constant. In this way you can easily multiply and get the right answer without changing the rate constant units.
- Thu Mar 12, 2020 10:31 am
- Forum: First Order Reactions
- Topic: equation derivations
- Replies: 9
- Views: 603
Re: equation derivations
I wouldnt be surprised to see a derivation on the exam. Some of the UAs had a derivation problem on their second test last year. Also lavelle specifically went through the derivations in class, so we should probably know how to do it.
- Thu Mar 12, 2020 10:29 am
- Forum: First Order Reactions
- Topic: Half Life Equations
- Replies: 10
- Views: 625
Re: Half Life Equations
For Zero Order reaction: t½ = [Ao] / 2k
For First Order reaction: t½ = 0.693 / k
For Second Order Reaction: t½ = 1 / k [Ao]
They are given, but they are not labeled on the constants sheet.
For First Order reaction: t½ = 0.693 / k
For Second Order Reaction: t½ = 1 / k [Ao]
They are given, but they are not labeled on the constants sheet.
- Thu Mar 12, 2020 10:25 am
- Forum: First Order Reactions
- Topic: 7A7
- Replies: 3
- Views: 307
Re: 7A7
For order zero, the rate constant has units of mol·L−1·s−1 (or M·s−1)
For order one, the rate constant has units of s−1
For order two, the rate constant has units of L·mol−1·s−1 (or M−1·s−1)
And for order three, the rate constant has units of L2·mol−2·s−1 (or M−2·s−1)
For order one, the rate constant has units of s−1
For order two, the rate constant has units of L·mol−1·s−1 (or M−1·s−1)
And for order three, the rate constant has units of L2·mol−2·s−1 (or M−2·s−1)
- Thu Mar 05, 2020 11:57 am
- Forum: Work, Gibbs Free Energy, Cell (Redox) Potentials
- Topic: Knowing If K>1 in Redox Reactions
- Replies: 3
- Views: 702
Re: Knowing If K>1 in Redox Reactions
When K > 1, the reaction is spontaneous because this makes delta G negative. So when the question asks to find if K > 1, it wants you to find the sign of delta G and thus if the reaction is spontaneous. You can easily find the sign of delta G, by finding the E^{o} of the cell. You can use the equat...
- Mon Mar 02, 2020 9:56 pm
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: 6N.13
- Replies: 2
- Views: 203
Re: 6N.13
You will need to use the nernst equation: ) . We are given E cell, and you can find the E standard from the half reactions from appendix 2b.
. We are given E cell, and you can find the E standard from the half reactions from appendix 2b.
- Sun Mar 01, 2020 7:26 pm
- Forum: Work, Gibbs Free Energy, Cell (Redox) Potentials
- Topic: Problem 6N.1 part b
- Replies: 4
- Views: 445
Re: Problem 6N.1 part b
I believe the solutions manual is wrong.
- Sun Mar 01, 2020 7:25 pm
- Forum: Work, Gibbs Free Energy, Cell (Redox) Potentials
- Topic: Free energy
- Replies: 3
- Views: 363
Re: Free energy
- Sun Mar 01, 2020 7:08 pm
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: Concentration Cell Examples
- Replies: 3
- Views: 287
Re: Concentration Cell Examples
A Concentration cell, is a like a galvanic cell except the chemical species on both sides is the same. If you wanted to find the E standard, it would be 0 because the 2 rxn are the same, and so the you would add the reduction E standard with the E standard of the oxidation, which is equal but opposi...
- Thu Feb 27, 2020 8:26 pm
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: 6N.13
- Replies: 2
- Views: 302
Re: 6N.13
Your work is right, I don't know why they rounded; It might just be a solution error
- Thu Feb 27, 2020 8:12 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Cell Diagram Aqueous Solutions Order
- Replies: 1
- Views: 211
Re: Cell Diagram Aqueous Solutions Order
I believe it can be either or. I always right in the way the reaction proceeds, so if Cr4+/Cr3+ are on the anode side, I would write the cell diagram 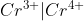
- Thu Feb 27, 2020 7:56 pm
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: log vs. ln in Nernst
- Replies: 2
- Views: 266
Re: log vs. ln in Nernst
This is also part of the reason for the pH scale. We take numbers like .001 and .0001 and say they are 1 pH value apart, while in reality, .001 is ten times greater than .0001. We use ln and log to view very large or very small numbers on an easier scale that we can easily interpret
- Thu Feb 27, 2020 7:51 pm
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: 6N.3 a)
- Replies: 1
- Views: 200
Re: 6N.3 a)
.075 Molar is part of the oxidation half reaction, but it ends up on the products side of the overall reaction.
- Thu Feb 27, 2020 7:49 pm
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: Concentration Cells
- Replies: 6
- Views: 536
Re: Concentration Cells
A concentration cell is an electrolytic cell that is comprised of two half-cells with the same electrodes, but differing in concentrations. The only work done from a concentration cell occurs from a difference in concentration
- Wed Feb 19, 2020 9:13 pm
- Forum: Balancing Redox Reactions
- Topic: Oxidation Numbers
- Replies: 7
- Views: 465
Re: Oxidation Numbers
If you know the oxidation numbers of a species on both sides of a chemical reaction, then you will know if it is reduced, stay the same, or oxidizedMaya Pakulski 1D wrote:What are oxidation numbers used for?
- Wed Feb 19, 2020 8:10 pm
- Forum: Balancing Redox Reactions
- Topic: What is being reduced/oxidized in this rxn?
- Replies: 4
- Views: 382
Re: What is being reduced/oxidized in this rxn?
Your Oxidation half-reaction is correct
- Wed Feb 19, 2020 8:03 pm
- Forum: Balancing Redox Reactions
- Topic: Homework 6K.3 d
- Replies: 1
- Views: 200
Re: Homework 6K.3 d
According to other forum posts, there is an error in the questions and it should be: 
- Wed Feb 19, 2020 8:00 pm
- Forum: Balancing Redox Reactions
- Topic: Oxidation Numbers
- Replies: 7
- Views: 465
Re: Oxidation Numbers
The oxidation state of oxygen in its compounds is usually -2, except for peroxides like H2O2, and Na2O2, in which the oxidation state for O is -1. The oxidation state of hydrogen is +1 in its compounds, except for metal hydrides, such as NaH, LiH, etc., in which the oxidation state for H is -1.
- Wed Feb 19, 2020 7:58 pm
- Forum: Balancing Redox Reactions
- Topic: Redox reactions
- Replies: 2
- Views: 195
Re: Redox reactions
Since we need to usually need to balance the reactions in a solution, we need to add water. By doing this you might need to then balance the Hydrogens, and to do this you add H+ and OH- based on the context of the question
- Fri Feb 14, 2020 6:00 pm
- Forum: Concepts & Calculations Using Second Law of Thermodynamics
- Topic: Delta S
- Replies: 2
- Views: 179
Re: Delta S
No work is done in free expansion because there is no opposing force. No work is done by pushing if there is nothing to push against, so w=0. Since its isothermal, so \Delta U = 0 . \Delta U = q+w , where 0 = q + 0, so q is also 0, if q is 0 , then \Delta S_{Surrondings} = 0 , because \Delta S = q_{...
- Fri Feb 14, 2020 3:27 pm
- Forum: Calculating Work of Expansion
- Topic: Reversible vs Irreversible
- Replies: 13
- Views: 900
Re: Reversible vs Irreversible
If a system is reversible that means that any 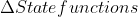 are equal to zero
are equal to zero
- Fri Feb 14, 2020 3:25 pm
- Forum: Entropy Changes Due to Changes in Volume and Temperature
- Topic: Cv vs Cp
- Replies: 17
- Views: 1005
Re: Cv vs Cp
Also remember that for Ideal gasses you can use the equation, Cpm = Cvm + R
- Wed Feb 12, 2020 12:02 am
- Forum: Administrative Questions and Class Announcements
- Topic: Pizza Rolls REVIEW Session DOWNLOAD HERE
- Replies: 67
- Views: 5975
Re: Pizza Rolls REVIEW Session DOWNLOAD HERE
My final equation for number 10 is (25_{grams}*2.01_{C_{Ice} }*T_{f}-0) + (6010_{\Delta Hfus_{Jules}}*1.38_{moles})= -(265_{grams}*4.184_{C_{Liquid}}*(T_{f}-25)) , but I keep getting the wrong degrees. What am I doing wrong? I realized that after the dHfus occurs, th...
- Tue Feb 11, 2020 10:29 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Pizza Rolls REVIEW Session DOWNLOAD HERE
- Replies: 67
- Views: 5975
Re: Pizza Rolls REVIEW Session DOWNLOAD HERE
For Question 5, I have ) for both gasses, but when I add them up I dont get the right answer. Is there another equation to find the change of Entropy of the 2 gasses combined, if so what is it?
for both gasses, but when I add them up I dont get the right answer. Is there another equation to find the change of Entropy of the 2 gasses combined, if so what is it?
- Tue Feb 11, 2020 10:09 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Pizza Rolls REVIEW Session DOWNLOAD HERE
- Replies: 67
- Views: 5975
Re: Pizza Rolls REVIEW Session DOWNLOAD HERE
My final equation for number 10 is  + (6010_{\Delta Hfus_{Jules}}*1.38_{moles})= -(265_{grams}*4.184_{C_{Liquid}}*(T_{f}-25))) , but I keep getting the wrong degrees. What am I doing wrong?
, but I keep getting the wrong degrees. What am I doing wrong?
- Thu Feb 06, 2020 12:36 pm
- Forum: Concepts & Calculations Using Second Law of Thermodynamics
- Topic: 4F17
- Replies: 1
- Views: 126
Re: 4F17
First you need to heat up the liquid water to 100 degrees and calculate entropy, this is the heating part. Then you need to vaporize, and use the given number in the question. Then you need to cool it back down to 85 degrees in order to correctly answer the question.
- Thu Feb 06, 2020 12:18 pm
- Forum: Thermodynamic Definitions (isochoric/isometric, isothermal, isobaric)
- Topic: ∆U When ∆T = 0
- Replies: 5
- Views: 390
Re: ∆U When ∆T = 0
- Wed Feb 05, 2020 11:59 pm
- Forum: Thermodynamic Systems (Open, Closed, Isolated)
- Topic: Insulated system
- Replies: 5
- Views: 300
Re: Insulated system
It always depends on the system's pressure and volume. In some situations You can use the equation 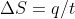 . If there is no heat transfer then q is 0, so delta S would be zero
. If there is no heat transfer then q is 0, so delta S would be zero
- Wed Feb 05, 2020 11:56 pm
- Forum: Thermodynamic Systems (Open, Closed, Isolated)
- Topic: Change in Internal Energy
- Replies: 6
- Views: 4581
Re: Change in Internal Energy
005384106 wrote:will we ever need to do any conversions for units? do these problems always need to be in kJ?
I assume that you will need to do unit conversions on the test to get the answer right. Its always easier to use the units that are in the questions.
- Wed Feb 05, 2020 11:53 pm
- Forum: Calculating Work of Expansion
- Topic: units
- Replies: 9
- Views: 260
Re: units
Also remember that degeneracy, Capital W, has no units. So for the equation ) , the units of boltzmann constant is the same as entropy
, the units of boltzmann constant is the same as entropy
- Thu Jan 30, 2020 1:51 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Bond enthalpy
- Replies: 2
- Views: 125
Re: Bond enthalpy
\Sigma \Delta H_(Bonds broken) - \Sigma \Delta H(Bondsformed) . You add up the bond enthalpies from the product side and subtract it from the sum of the bond enthalpies of the reactant side. Also remember to take into account the stoichometric values in your math. You are right that...
- Thu Jan 30, 2020 1:41 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Standard Reaction Enthalpies
- Replies: 2
- Views: 106
Re: Standard Reaction Enthalpies
I would say that you should be able to use all 3, since the problem could give you constants and values that only one of the methods can utilize
- Tue Jan 28, 2020 10:37 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: 4E.5, 4E.7
- Replies: 7
- Views: 629
Re: 4E.5, 4E.7
For 4.E.5, Looking at the lewis structure for C2H2 gives us 1 C-C triple bond, which has an enthalpy of 837, and also 2 C-H bonds which have a combined value of 824. Add these 2 numbers and multiply by the moles, which is 3, and you get the total enthalpy for C2H2, 4983. For benzene, the table of bo...
- Tue Jan 28, 2020 8:04 pm
- Forum: Phase Changes & Related Calculations
- Topic: Change in Temperature
- Replies: 10
- Views: 306
Re: Change in Temperature
You can think of this in another way: imagine putting heat as a reactant or product based on if the reaction is endothermic or exothermic. Then you can use le chatelier's principle to determine how K will change. For an exothermic reaction, the heat is on the products side because it is released. If...
- Tue Jan 28, 2020 7:57 pm
- Forum: Phase Changes & Related Calculations
- Topic: Cv and Cp
- Replies: 9
- Views: 458
Re: Cv and Cp
Do solids and liquids only use C? Or do they use Cv? I'm pretty sure they don't use Cp. They are the same for solids and liquids. For gasses, they are easily compressible, meaning their specific volume can easily change, while solids and liquids cannot change volume that easily so we just say that ...
- Thu Jan 23, 2020 6:29 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Difference Kw Value
- Replies: 3
- Views: 382
Re: Difference Kw Value
I believe that anytime the the concentration of H30+ = concentration OH- is considered neutral even if the ph isn't 7, which is neutral for water at room temperature. If kw changes because of a change in temperature then we should expect the ph scale and what is acidic, neutral, and basic should al...
- Thu Jan 23, 2020 4:51 pm
- Forum: Phase Changes & Related Calculations
- Topic: Water Phases
- Replies: 2
- Views: 150
Re: Water Phases
Yes, 500 cal/g is the middle point of a phase change from liquid to vapor. Half of the water would be in the liquid state and the other half would be in the vapor state. It is also important to understand that the temperature is not changing during a phase change because all of the heat is going to...
- Wed Jan 22, 2020 11:22 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Acidity Constant
- Replies: 4
- Views: 113
Re: Acidity Constant
A strong Acid(high Ka) has a weak conjugate base(Low Kb). A strong Base(High Kb has a weak conjugate acid(Low Ka).
- Wed Jan 22, 2020 10:39 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Test 1 Practice Worksheet #5
- Replies: 6
- Views: 276
Re: Test 1 Practice Worksheet #5
Jessica Li 4F wrote:I actually got 8.6 - I think you forgot to convert the given pKa value to pKb, as the molecule you are dealing with is actually the base.
I got the same answer as you!
- Wed Jan 22, 2020 9:37 pm
- Forum: Non-Equilibrium Conditions & The Reaction Quotient
- Topic: 6B 9
- Replies: 3
- Views: 225
Re: 6B 9
Any [H30+] over 1 will have a negative pH, because the -log(1)= 0, because 10^0=1. For example if the molarity is 2, to find the pH you should do -log(2), which is equal to -.3
- Wed Jan 22, 2020 9:31 pm
- Forum: Phase Changes & Related Calculations
- Topic: Water Phases
- Replies: 2
- Views: 150
Water Phases
https://opentextbc.ca/physicstestbook2/wp-content/uploads/sites/211/2017/10/Figure_15_03_03a.jpg
This is a graph similar to what we saw in class today.
At ~500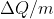 does that mean around 50% of the water is in liquid state and the other half in vapor state?
does that mean around 50% of the water is in liquid state and the other half in vapor state?
This is a graph similar to what we saw in class today.
At ~500
- Tue Jan 21, 2020 9:11 pm
- Forum: Ideal Gases
- Topic: homework #3
- Replies: 16
- Views: 968
Re: homework #3
You can do the homework on any content that we discussed in class. For the first 2 weeks, We've gone over the first 2 sections, and in Week 3 we will most likely move on to Outline 3, so you could potentially do problems from that set. If you want practice before the test you could do your homework ...
- Tue Jan 21, 2020 9:08 pm
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Difference in PH between strong and weak acids
- Replies: 11
- Views: 577
Re: Difference in PH between strong and weak acids
The strong acids have a lower pH because they fully dissociate and have a lower hydronium concentration. Weak acids are the opposite. You are right that strong acids has a lower pH because they fully dissociate, but they have a higher hydronium concentration. The more H3O+ you have, the lower on th...
- Tue Jan 21, 2020 7:56 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Calculator
- Replies: 4
- Views: 194
Calculator
Is the TI-36X an acceptable calculator for this course?
- Thu Jan 16, 2020 6:53 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: pOH
- Replies: 1
- Views: 123
pOH
Calculate the molar concentration of OH- in solutions with the following molar concentrations of H3O+. c: 3.1mol/L First I calculate pH of it, using -log(3.1)=-.49. Then i use the equation pKw = pH+pOH, also written as 14= -.49 + x. I solve for x and get 14.49. Then i do 10^(-14.49) to find the conc...
- Thu Jan 16, 2020 4:13 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Difference Kw Value
- Replies: 3
- Views: 382
Re: Difference Kw Value
I believe that anytime the the concentration of H30+ = concentration OH- is considered neutral even if the ph isn't 7, which is neutral for water at room temperature. If kw changes because of a change in temperature then we should expect the ph scale and what is acidic, neutral, and basic should all...
- Thu Jan 16, 2020 4:09 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: 6D.13
- Replies: 4
- Views: 121
Re: 6D.13
You should definitly know some strong acids, and even further you should know what the difference between strong acids and weak acids.
- Thu Jan 16, 2020 3:18 pm
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Inert Gas and Le Chatliers
- Replies: 7
- Views: 277
Re: Inert Gas and Le Chatliers
If you had Cl- as an inert gas it would be Cl- on the reactant side and Cl- on the product side. If you add this into the chemical equilibrium constant equation, the concentration of Cl- will be on the top and bottom of the division sign, so they will simply cancel each other out.
- Tue Jan 14, 2020 12:39 pm
- Forum: Ideal Gases
- Topic: Variables
- Replies: 3
- Views: 130
Re: Variables
If a question gives you concentration you can still use the equation, because (n/V)= concentration, where n is number of moles and V is volume. moles/volume is molarity and that is concentration
- Tue Jan 14, 2020 12:35 pm
- Forum: Ideal Gases
- Topic: ICE tables
- Replies: 9
- Views: 1518
Re: ICE tables
If the questions asks for just K, and not Kc or Kp, then you need to look at the equation. If the reactants and products are gases then you should use Kp, because it is implied. If the reactants and products of the equation are aqueous then you should use Kc because it is implied. However if it aske...
- Thu Jan 09, 2020 8:23 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Solutions Manual
- Replies: 4
- Views: 250
Solutions Manual
Hi,
I was wondering if I had access to the solutions manual for my textbook. I bought the sapling learning pack that included the textbook, but I cannot seem to find the solutions manual. Please help.
I was wondering if I had access to the solutions manual for my textbook. I bought the sapling learning pack that included the textbook, but I cannot seem to find the solutions manual. Please help.
- Thu Jan 09, 2020 6:52 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: 5I.17
- Replies: 3
- Views: 531
Re: 5I.17
I'm not sure how you got x=0.0114, but if you plug in all the values into the K c ratio so that it's 1.00*10 -5 =(2x) 2 /((0.114-x)(0.114-x)) and solve for x using the quadratic formula, you should get x=1.8*10 -4 . I did 1.00x10^-5=(2x)^2/(0.114)^2 instead of (0.114-x)(0.114-x) for the denominator...
- Thu Jan 09, 2020 6:45 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Temperature's affects on chemical equilibria
- Replies: 2
- Views: 106
Re: Temperature's affects on chemical equilibria
I believe your question will be adressed in thermodynamics, with equations like 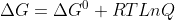 , where we are able to calculate the effect of temperature on a reaction
, where we are able to calculate the effect of temperature on a reaction
- Thu Jan 09, 2020 6:40 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Ratios of Pressure
- Replies: 2
- Views: 111
Ratios of Pressure
A sample of ozone,O3, amounting to 0.10 mol, is placed in a sealed container of volume 1.0 L and the reaction 2O_{3}(g)\rightarrow 3O_{2}(g) is allowed to reach equilibrium.Then 0.50 mol O3 is placed in a second container of volume 1.0 L at the same temperature and allowed to reach e...
- Wed Jan 08, 2020 4:22 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Hw problem 5G.5
- Replies: 1
- Views: 86
Re: Hw problem 5G.5
A quick note, this problem is not listed as a homework problem. For 5G it says Problems 5G: 1, 3, 7, 9, 11;
- Wed Jan 08, 2020 4:10 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Partial Pressure
- Replies: 3
- Views: 204
Partial Pressure
A sample of ozone,O3, amounting to 0.10 mol, is placed in a sealed container of volume 1.0 L and the reaction 2O_{3}(g)\rightarrow 3O_{2} is allowed to reach equilibrium.Then 0.50 mol O3 is placed in a second container of volume 1.0 L at the same temperature and allowed to reach equilibrium....

