Search found 50 matches
- Sat Mar 14, 2020 3:53 pm
- Forum: First Order Reactions
- Topic: First order rxns
- Replies: 6
- Views: 427
Re: First order rxns
you can look at the rate law given or graph
- Sat Mar 14, 2020 3:50 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Final
- Replies: 12
- Views: 828
Re: Final
I think electronically, probably scan and submit?
- Sat Mar 14, 2020 3:49 pm
- Forum: Administrative Questions and Class Announcements
- Topic: take home FINAL DEADLINE
- Replies: 15
- Views: 1124
Re: take home FINAL DEADLINE
Honestly, I'm still waiting for the email that he said he will send today. I understand that he has been busy, but hopefully itll come soon because im getting anxious
- Sat Mar 14, 2020 3:46 pm
- Forum: Administrative Questions and Class Announcements
- Topic: "Open Book" Final?
- Replies: 30
- Views: 2090
Re: "Open Book" Final?
im thinking textbook, lecture notes, discussion notes
- Sat Mar 14, 2020 3:44 pm
- Forum: Arrhenius Equation, Activation Energies, Catalysts
- Topic: Activation energy
- Replies: 7
- Views: 476
Re: Activation energy
joules or kilojoules
- Sat Mar 14, 2020 3:41 pm
- Forum: *Nucleophiles
- Topic: FInal
- Replies: 11
- Views: 1720
Re: FInal
I think it would be best to
- Sat Mar 14, 2020 3:40 pm
- Forum: Balancing Redox Reactions
- Topic: Oxidation number
- Replies: 12
- Views: 1230
Re: Oxidation number
determining the oxidation number determines whether it is oxidizing or reducing. when balancing half reactions, it will show how many electrons will be lost or gained
- Sat Mar 14, 2020 3:36 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Cell Diagram
- Replies: 11
- Views: 746
Re: Cell Diagram
yes, it is probably the most common
- Sat Mar 14, 2020 3:35 pm
- Forum: Zero Order Reactions
- Topic: 0 order
- Replies: 6
- Views: 503
Re: 0 order
Concentration does not affect rate in zero order reaction
- Sat Mar 14, 2020 3:34 pm
- Forum: Administrative Questions and Class Announcements
- Topic: final tech malfunctions
- Replies: 5
- Views: 461
Re: final tech malfunctions
they will probably have us download and print the finals
- Sat Mar 14, 2020 3:33 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Partial Credit
- Replies: 8
- Views: 580
Re: Partial Credit
you can just an app call genius scan for android users to scan
- Sat Mar 14, 2020 3:31 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Final- general questions
- Replies: 12
- Views: 938
Re: Final- general questions
wait so is the exam three hours or will he just post it tomorrow? I just want to know because other professor have given a twenty-four hours limit
- Mon Mar 02, 2020 8:05 am
- Forum: Method of Initial Rates (To Determine n and k)
- Topic: Determining n
- Replies: 5
- Views: 471
Re: Determining n
n is the number of moles of e in the reaction that is being transferred
- Mon Mar 02, 2020 8:04 am
- Forum: Kinetics vs. Thermodynamics Controlling a Reaction
- Topic: Kinetics in Test 2?
- Replies: 13
- Views: 826
Re: Kinetics in Test 2?
no, that is the only topic that will not be tested before the final
- Mon Mar 02, 2020 8:03 am
- Forum: Thermodynamic Systems (Open, Closed, Isolated)
- Topic: Kelvin or Celsius?
- Replies: 86
- Views: 5656
Re: Kelvin or Celsius?
kelvin, just make sure the units for R is the same
- Mon Mar 02, 2020 8:02 am
- Forum: Method of Initial Rates (To Determine n and k)
- Topic: k
- Replies: 10
- Views: 609
Re: k
k is unit-less because it is a ration of concentrations of products and reactants
- Mon Mar 02, 2020 8:01 am
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Difference Between Galvanic and Voltaic Cells
- Replies: 3
- Views: 282
Re: Difference Between Galvanic and Voltaic Cells
same kind of cells, just different names
- Mon Mar 02, 2020 8:00 am
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: predicting is metals will dissolve
- Replies: 3
- Views: 227
Re: predicting is metals will dissolve
identify delta g- spontaneous--> will dissolves
not spontaneous--> will not dissolves
not spontaneous--> will not dissolves
- Mon Mar 02, 2020 7:58 am
- Forum: Balancing Redox Reactions
- Topic: Anode and Xathod
- Replies: 9
- Views: 548
Re: Anode and Xathod
on the left side, oxidation is taking place at the anode- so the rod itself is giving off electrons which then travel to the right side, the cathode to binds with free floating molecules waiting to be reduced.
- Mon Mar 02, 2020 7:56 am
- Forum: Work, Gibbs Free Energy, Cell (Redox) Potentials
- Topic: Voltage Signs for Oxidation Reactions
- Replies: 2
- Views: 168
Re: Voltage Signs for Oxidation Reactions
yes, just change the sign of the given value
- Mon Mar 02, 2020 7:55 am
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: salt bridge
- Replies: 4
- Views: 348
Re: salt bridge
the salt bridge is needed to maintain the charge balance of the two solutions as the electrons move from the anode to cathode, it leaves the left-side solution positively charged, therefore the ions from the salt bridge enters to to stabilize the charge difference
- Mon Mar 02, 2020 7:52 am
- Forum: Work, Gibbs Free Energy, Cell (Redox) Potentials
- Topic: work
- Replies: 7
- Views: 450
Re: work
delta g is equal to the max non-expansion work (at constant p and t)
- Mon Mar 02, 2020 7:48 am
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Voltage
- Replies: 6
- Views: 438
Re: Voltage
the sign is used more to keep track, so its a good idea to use it as it minimizes your chances of making a mistake
- Mon Mar 02, 2020 7:46 am
- Forum: Method of Initial Rates (To Determine n and k)
- Topic: factors that affect k
- Replies: 8
- Views: 709
Re: factors that affect k
the equilibrium changes in responds to change in temperature
- Mon Mar 02, 2020 7:42 am
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Stoichiometric Coefficients in Cell Diagrams
- Replies: 3
- Views: 227
Re: Stoichiometric Coefficients in Cell Diagrams
you don't include the coefficients in the cell diagrams
- Mon Mar 02, 2020 7:38 am
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: Moles in nernst
- Replies: 4
- Views: 311
Re: Moles in nernst
n refers to the moles of electrons that is being transfered
- Mon Mar 02, 2020 7:36 am
- Forum: Interesting Applications: Rechargeable Batteries (Cell Phones, Notebooks, Cars), Fuel Cells (Space Shuttle), Photovoltaic Cells (Solar Panels), Electrolysis, Rust
- Topic: biological examples
- Replies: 3
- Views: 360
Re: biological examples
not sure if this answers your question, but electricity is used in the body so now physicians use EEG to detect abnormalities
- Mon Mar 02, 2020 7:32 am
- Forum: Kinetics vs. Thermodynamics Controlling a Reaction
- Topic: Study Advice
- Replies: 73
- Views: 7084
Re: Study Advice
personally, lyndon's reviews are great for me.
- Wed Feb 05, 2020 5:31 pm
- Forum: Thermodynamic Systems (Open, Closed, Isolated)
- Topic: heat given off by rxn = - heat absorbed by solution
- Replies: 6
- Views: 328
Re: heat given off by rxn = - heat absorbed by solution
I don't think it is important to memorize that equation. As long as you understand that losing heat is neg and absorbing is pos. and the amount of energy lost/gain by the system is equal to the amount gain/lost by the surrounding.
- Wed Feb 05, 2020 5:19 pm
- Forum: Thermodynamic Systems (Open, Closed, Isolated)
- Topic: enthalpy
- Replies: 7
- Views: 248
Re: enthalpy
Enthalpy is the amount of heat released or absorbed when the rxn is at a constant pressure
- Wed Feb 05, 2020 5:16 pm
- Forum: Calculating Work of Expansion
- Topic: Kelvin vs Celsius
- Replies: 5
- Views: 176
Re: Kelvin vs Celsius
It would depends on the problems, one of the best thing to do is check the units of a given constant in that problem.
- Wed Feb 05, 2020 5:15 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: defintion
- Replies: 6
- Views: 380
Re: defintion
Internal energy is the total energy found in a system which is, as defined by the equation, the sum of heat plus work (a form of energy)
- Wed Feb 05, 2020 5:09 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: calculating q: moles or mass?
- Replies: 2
- Views: 272
Re: calculating q: moles or mass?
It can be either one depending on the units of the C that you use
- Wed Feb 05, 2020 5:05 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Cp and Cv
- Replies: 10
- Views: 479
Re: Cp and Cv
I don't think we have to memorize them
- Sun Jan 26, 2020 9:23 pm
- Forum: Non-Equilibrium Conditions & The Reaction Quotient
- Topic: Using Kc Vs Kp
- Replies: 22
- Views: 1061
Re: Using Kc Vs Kp
It would depends on the chemical equation given
- Sun Jan 26, 2020 9:22 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Kp to Kc
- Replies: 11
- Views: 594
Re: Kp to Kc
yes it is included in the calculation as long as it is not in pure liquid form
- Sun Jan 26, 2020 9:20 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Percent Ionization
- Replies: 12
- Views: 567
Re: Percent Ionization
Percent Ionization = ([H3O+]/[initial concentration of acid]) x 100
- Sun Jan 26, 2020 9:18 pm
- Forum: Calculating Work of Expansion
- Topic: Work Units
- Replies: 3
- Views: 131
Re: Work Units
I believe that it is measured in joules
- Sun Jan 26, 2020 9:11 pm
- Forum: Phase Changes & Related Calculations
- Topic: enthalpy of phase changes
- Replies: 8
- Views: 261
Re: enthalpy of phase changes
I do not believe that enthalpy values will ever be negative
- Sun Jan 26, 2020 9:10 pm
- Forum: Phase Changes & Related Calculations
- Topic: Physical or Phase Changes
- Replies: 7
- Views: 192
Re: Physical or Phase Changes
Following the examples that have already been given- those values will most likely be given to us
- Sat Jan 18, 2020 12:30 pm
- Forum: Administrative Questions and Class Announcements
- Topic: How was my Friday class/lecture?
- Replies: 4
- Views: 249
Re: How was my Friday class/lecture?
We went over some examples as practice. No new material
- Sat Jan 18, 2020 12:28 pm
- Forum: Non-Equilibrium Conditions & The Reaction Quotient
- Topic: Partial Pressure
- Replies: 19
- Views: 745
Re: Partial Pressure
Partial pressure (from what I have seen) is usually given in the problem. But I do not doubt that we might have to solve for Partial Pressure in some cases.
- Sat Jan 18, 2020 12:26 pm
- Forum: Ideal Gases
- Topic: Topics on Test 1
- Replies: 37
- Views: 1385
Re: Topics on Test 1
Test one will be covering Chemical Equilibrium and Acid&Base Equilibria
- Sat Jan 18, 2020 12:25 pm
- Forum: Non-Equilibrium Conditions & The Reaction Quotient
- Topic: Q < K
- Replies: 16
- Views: 842
Re: Q < K
When Q < K, that means the reaction is not in equilibrium and the reaction is favoring the product side.
- Sat Jan 18, 2020 12:22 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Study Guide Test One
- Replies: 17
- Views: 686
Re: Study Guide Test One
I do not think that there will be a study guide for test one, but Professor Lavelle said that the extra problems that he put on the outlines are good practice.
- Mon Jan 13, 2020 6:24 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: 6B.3
- Replies: 2
- Views: 73
Re: 6B.3
I'm having the same problem. Please help
- Mon Jan 13, 2020 6:19 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Autoprotolysis
- Replies: 6
- Views: 372
Re: Autoprotolysis
Autoprotolysis can happen with other molecules apart from water molecules, such as ammonia and acetic acid.
- Fri Jan 10, 2020 2:48 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Meaning of equilibrium constant
- Replies: 5
- Views: 246
Re: Meaning of equilibrium constant
When K is between 10^-3 and 10^3, it means that neither the reactants nor the products are favored when the reaction is in equilibrium. You can't necessarily tell which side of the equilibrium has more in concentrations.
- Fri Jan 10, 2020 2:43 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Textbook 5H.1 Part 1
- Replies: 4
- Views: 158
Re: Textbook 5H.1 Part 1
We get the 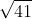
because when you write out the expression for K. We notice that the exponents (which are the coefficients in the equilibrium equation) are divided by 2. Therefore, in order to keep the two sides equal, we must take the Square root of 41.
because when you write out the expression for K. We notice that the exponents (which are the coefficients in the equilibrium equation) are divided by 2. Therefore, in order to keep the two sides equal, we must take the Square root of 41.
- Fri Jan 10, 2020 2:29 am
- Forum: Ideal Gases
- Topic: Ideal Gases
- Replies: 7
- Views: 535
Re: Ideal Gases
Although gases are never perfectly "ideal". We assume most of the time that gases are ideal because at higher temperature and lower pressure there are not a significant intermolecular forces between the gas molecules. This assumption allows for calculations of other properties of the gases.

