Search found 102 matches
- Thu Mar 12, 2020 8:19 am
- Forum: Balancing Redox Reactions
- Topic: Determining the oxidizer and reducer
- Replies: 10
- Views: 744
Re: Determining the oxidizer and reducer
In determining the oxidizing agent and reducing agent, I would suggest figuring out the various oxidation states first.
- Thu Mar 12, 2020 8:11 am
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: How do you know a cell can do work?
- Replies: 7
- Views: 515
Re: How do you know a cell can do work?
When E cell is equal to zero, the cell cannot do work.
- Thu Mar 12, 2020 8:09 am
- Forum: Kinetics vs. Thermodynamics Controlling a Reaction
- Topic: Units
- Replies: 8
- Views: 2893
Re: Units
As this visual shows, the units for the rate constant change, but the units for the rate remain the same.

- Thu Mar 12, 2020 8:05 am
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Lavelle's review slides
- Replies: 3
- Views: 363
Re: Lavelle's review slides
Since neither Br(aq) and Br(g) are conducting solids, Pt(s) has to be included in the cell diagram.
- Thu Mar 12, 2020 8:01 am
- Forum: *Enzyme Kinetics
- Topic: How to distinguish the intermediates and catalysts?
- Replies: 9
- Views: 879
Re: How to distinguish the intermediates and catalysts?
This video might help you understand how to distinguish between intermediates and catalysts:
https://www.brightstorm.com/science/chemistry/chemical-reaction-rates/tips-on-differentiating-between-a-catalyst-and-an-intermediate/
https://www.brightstorm.com/science/chemistry/chemical-reaction-rates/tips-on-differentiating-between-a-catalyst-and-an-intermediate/
- Sun Mar 08, 2020 9:41 am
- Forum: Reaction Mechanisms, Reaction Profiles
- Topic: the intermediate in a reaction
- Replies: 10
- Views: 710
Re: the intermediate in a reaction
The intermediate is not included in the rate law expression. You can recognize a species is an intermediate if it is in the steps, but not included in the overall reaction.
- Sun Mar 08, 2020 9:37 am
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: Calculating ln Q
- Replies: 20
- Views: 1606
Re: Calculating ln Q
When calculating Q for the Nernst equation, you can use both partial pressure and concentration. Before, all the values plugged into Q had to be all concentration values or partial pressure values.
- Sun Mar 08, 2020 9:33 am
- Forum: Kinetics vs. Thermodynamics Controlling a Reaction
- Topic: instantaneous rate
- Replies: 16
- Views: 973
Re: instantaneous rate
With average rate, there is a larger error because you are treating the curve as a line when taking two points into consideration. However, with instantaneous rate, you are using only one point to get the slope, which results in a more accurate slope.
- Sun Mar 08, 2020 9:28 am
- Forum: Balancing Redox Reactions
- Topic: Cathode and Anode
- Replies: 24
- Views: 1632
Re: Cathode and Anode
My TA mentioned that E cell can be negative sometimes and I believe there is a question with a negative E cell in section 6N.
- Sun Mar 08, 2020 9:25 am
- Forum: Method of Initial Rates (To Determine n and k)
- Topic: factors that affect k
- Replies: 8
- Views: 713
Re: factors that affect k
CMaduno_1L wrote:if you are referring to the reaction rate constant (lowercase k), then I believe that varying the temperature and solvent would affect its value.
An increase in temperature causes an increase in the rate constant (k) and a decrease in temperature causes a decrease in the rate constant (k).
- Sat Feb 29, 2020 2:37 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Galvanic vs Voltaic Cells
- Replies: 4
- Views: 915
Re: Galvanic vs Voltaic Cells
Galvanic and voltaic cells are the same thing, but with different names. However, in comparison to galvanic cells, electrolytic cells convert electrical energy into chemical energy while galvanic cells convert chemical energy into electrical energy. Here's a chart that shows the key difference betw...
- Sat Feb 29, 2020 2:32 pm
- Forum: Balancing Redox Reactions
- Topic: Cell Diagrams
- Replies: 14
- Views: 988
Re: Cell Diagrams
In a cell diagram, the anode (oxidation reaction) is on the left and the cathode (reduction reaction) is on the right.
- Sat Feb 29, 2020 2:30 pm
- Forum: Work, Gibbs Free Energy, Cell (Redox) Potentials
- Topic: E potentials
- Replies: 5
- Views: 443
Re: E potentials
One of the UAs said that the values that will be given to us will be reduction potentials, not oxidation potentials.
- Sat Feb 29, 2020 2:28 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Anode vs Cathode
- Replies: 15
- Views: 875
Re: Anode vs Cathode
Bryce Barbee wrote:During discussion, my TA would tell us which one was the anode and which was the cathode. I think it is based off of left and right usually though.
My TA also would tell us which one was the anode and which one was the cathode.
- Sat Feb 29, 2020 2:25 pm
- Forum: Balancing Redox Reactions
- Topic: Test 2
- Replies: 19
- Views: 988
Re: Test 2
On the class website, it says that "Test 2 covers 2nd page of Outline 4: Thermodynamics and Outline 5: Electrochemistry and its Applications."
- Sat Feb 22, 2020 3:49 pm
- Forum: Balancing Redox Reactions
- Topic: Finding moles of the reaction
- Replies: 4
- Views: 529
Re: Finding moles of the reaction
In this example, 1 mole of electrons is transferred:
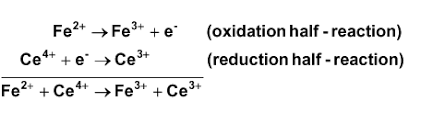
- Sat Feb 22, 2020 3:45 pm
- Forum: Balancing Redox Reactions
- Topic: Homework 6K1
- Replies: 4
- Views: 386
Re: Homework 6K1
C2H5OH and C2H4O have a net charge of zero, but (Cr2O7)-2 has a net charge of -2 and (Cr)3+ has a net charge of +3.
- Sat Feb 22, 2020 3:37 pm
- Forum: Van't Hoff Equation
- Topic: Van't Hoff Equation
- Replies: 3
- Views: 290
Re: Van't Hoff Equation
This image might help you see the correlations better:
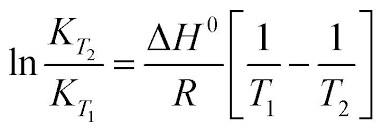
- Sat Feb 22, 2020 3:33 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: 5J.15
- Replies: 2
- Views: 314
Re: 5J.15
To calculate K, you need to manipulate the first equation in this image to the last equation:


- Sat Feb 22, 2020 3:29 pm
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Adding Inert Gas
- Replies: 20
- Views: 1133
Re: Adding Inert Gas
Increasing the pressure of a system with an inert gas does not affect the equilibrium constant. Some examples of inert gases are Helium (He), Argon (Ar), Neon (Ne), Krypton (Kr), and Xenon (Xe).
- Sat Feb 15, 2020 7:43 pm
- Forum: Thermodynamic Definitions (isochoric/isometric, isothermal, isobaric)
- Topic: Isothermal
- Replies: 9
- Views: 540
Re: Isothermal
To add to the above statement, the energy lost by the system doing work is replaced by heat flow, q, into the system, which is why q=-w.
- Sat Feb 15, 2020 7:39 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: When to use internal energy equation
- Replies: 3
- Views: 334
Re: When to use internal energy equation
In lecture, Dr. Lavelle mentioned this equation while explaining how a system loses energy as a result of doing work of expansion.
- Sat Feb 15, 2020 7:31 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: Delta U
- Replies: 8
- Views: 585
Re: Delta U
Changes in internal energy are a function of initial and final states. When delta U is zero, a gas still can expand because it is a spontaneous process with an entropy increase.
- Sat Feb 15, 2020 7:23 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: delta U= delta H
- Replies: 21
- Views: 1591
Re: delta U= delta H
When the volume of the reactants is equal to the volume of the products, delta U is equal to delta H.
- Sat Feb 15, 2020 7:14 pm
- Forum: Thermodynamic Definitions (isochoric/isometric, isothermal, isobaric)
- Topic: Isobaric systems
- Replies: 16
- Views: 845
Re: Isobaric systems
Leslie Almaraz 4G wrote:What about an adiabatic system? is there a different equation for that?
In an adiabatic system, there is no heat transfer (q=0).
- Fri Feb 07, 2020 7:17 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: negative Delta U
- Replies: 5
- Views: 315
Re: negative Delta U
A negative delta U occurs when the final internal energy is lower than the initial internal energy.
- Fri Feb 07, 2020 7:12 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: GFE dividing by temp
- Replies: 3
- Views: 177
Re: GFE dividing by temp
We know if it is a spontaneous process if the delta S of the universe is positive, which means that the delta G of the system must be negative.
- Fri Feb 07, 2020 7:10 pm
- Forum: Thermodynamic Systems (Open, Closed, Isolated)
- Topic: Isolated vs Closed [ENDORSED]
- Replies: 34
- Views: 4316
Re: Isolated vs Closed [ENDORSED]
Here's a visual that might help you:
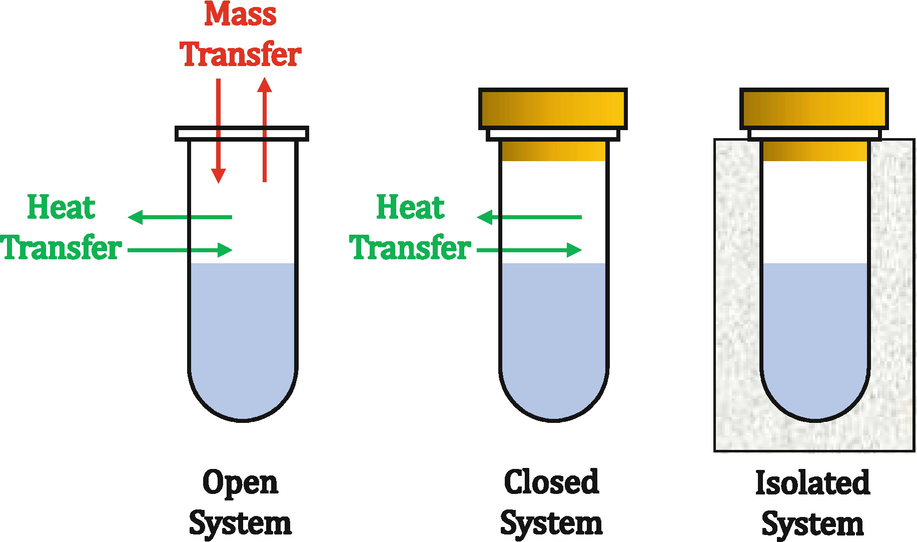

- Fri Feb 07, 2020 7:03 pm
- Forum: Phase Changes & Related Calculations
- Topic: Homework 4C 13
- Replies: 7
- Views: 380
Re: Homework 4C 13
The substance with a greater temperature (hotter) will be the one transferring heat to the substance with a lower temperature (colder), which is why the negative sign will be given to the water (which is losing its heat).


- Fri Feb 07, 2020 6:55 pm
- Forum: Phase Changes & Related Calculations
- Topic: Negative Work
- Replies: 18
- Views: 1491
Re: Negative Work
Here's a visual that shows an example of when work is negative:

- Sat Feb 01, 2020 12:40 pm
- Forum: Thermodynamic Systems (Open, Closed, Isolated)
- Topic: Isolated systems
- Replies: 4
- Views: 255
Re: Isolated systems
In a closed system, the system can exchange energy with the surroundings. In an isolated system, nothing exchanges with the surroundings, which means that we assume the insulation is effective and there is no energy being exchanged.
- Sat Feb 01, 2020 12:32 pm
- Forum: Concepts & Calculations Using Second Law of Thermodynamics
- Topic: Favored or Spontaneous
- Replies: 2
- Views: 131
Re: Favored or Spontaneous
A spontaneous reaction is a reaction that occurs in a given set of conditions without intervention. In lecture, Dr. Lavelle said that a spontaneous reaction means it is a favorable reaction.
- Sat Feb 01, 2020 12:17 pm
- Forum: Phase Changes & Related Calculations
- Topic: Phase Changes
- Replies: 17
- Views: 735
Re: Phase Changes
If you look at a heating curve, the slanted lines represent the temperature increasing, and the flat lines represent the phase change where there is no change in temperature. For example, on the first straight line, the leftmost point represents solid water at 0 degrees celsius, and on the most rig...
- Sat Feb 01, 2020 12:10 pm
- Forum: Concepts & Calculations Using Second Law of Thermodynamics
- Topic: Thermodynamic
- Replies: 1
- Views: 102
Re: Thermodynamic
Delta H is the change in enthalpy, delta U is the change in internal energy of the system, and delta S is related to entropy. The delta U (or delta H) and delta S of a system provides information on how the system will change.
- Sat Feb 01, 2020 12:03 pm
- Forum: Phase Changes & Related Calculations
- Topic: Calorimeter
- Replies: 8
- Views: 581
Re: Calorimeter
This is what a bomb calorimeter looks like: https://chem.libretexts.org/@api/deki/files/58277/c3f2def4f1ad04996c40999978baa644.jpg?revision=1&size=bestfit&width=435 This is what a calorimeter looks like: https://www.chem.fsu.edu/chemlab/chm1045/cpcalorimeter.png
- Sat Jan 25, 2020 11:55 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Q and K
- Replies: 4
- Views: 164
Re: Q and K
If K is small (K< 10^-3), we know that there are more reactants at equilibrium ("equilibrium sits to the left"). If K=1, both reactions are equally favorable, which is rare. If K is large (K> 10^3), there are more products at equilibrium ("equilibrium sits to the right"). For int...
- Sat Jan 25, 2020 11:48 am
- Forum: Phase Changes & Related Calculations
- Topic: Proton transfer in water
- Replies: 3
- Views: 184
Re: Proton transfer in water
This visual shows how the proton transfer occurs between two water molecules:

- Sat Jan 25, 2020 11:42 am
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: state functions
- Replies: 4
- Views: 1059
Re: state functions
These are all the state functions that Dr. Lavelle mentioned during lecture: energy, pressure, volume, temperature, density, and heat capacity.
- Sat Jan 25, 2020 11:39 am
- Forum: Phase Changes & Related Calculations
- Topic: Endothermic and Exothermic
- Replies: 13
- Views: 624
Re: Endothermic and Exothermic
Endothermic reactions use heat as a reactant because they require energy. Exothermic reactions use heat as a product because they release energy. This might be a helpful visual to see the role heat/energy plays in endothermic and exothermic reactions: https://i.ytimg.com/vi/APlWB5e2AP8/maxresdefaul...
- Sat Jan 25, 2020 11:32 am
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Hess's Law
- Replies: 5
- Views: 158
Re: Hess's Law
In the textbook, it says Hess's law states that "the overall reaction enthalpy is the sum of the reaction enthalpies of the steps into which the reaction can be divided."
- Sat Jan 18, 2020 12:30 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Peer Learning Worksheets
- Replies: 3
- Views: 203
Re: Peer Learning Worksheets
In the step up sessions I have attended, the UAs usually write out the questions for students to solve on the board instead of handing out worksheets or posting the questions online. I think the only time worksheets are handed out are in the workshops on Mondays, but they are not posted online.
- Sat Jan 18, 2020 12:13 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Study Guide Test One
- Replies: 17
- Views: 696
Re: Study Guide Test One
Use these Learning Outcomes to prepare for Test 1: Outline 1: Chemical Equilibrium https://lavelle.chem.ucla.edu/wp-content/supporting-files/Chem14B/Chem14B_Outline1_Chemical_Equilibrium.pdf Outline 2: Acids and Bases https://lavelle.chem.ucla.edu/wp-content/supporting-files/Chem14B/Chem14B_Outline...
- Sat Jan 18, 2020 12:10 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: 5I 25
- Replies: 4
- Views: 124
Re: 5I 25
It is necessary to find Q before K because we need to discern whether the forward or reverse reaction is favored. For this question, Q=0.75 and, since Q < K, we know the forward reaction is favored.
- Sat Jan 18, 2020 12:04 pm
- Forum: Non-Equilibrium Conditions & The Reaction Quotient
- Topic: Q < K
- Replies: 16
- Views: 848
Re: Q < K
When Q < K at some time during the reaction, then [R] > [P] and the forward reaction is favored (which is the same thing as saying it "proceeds towards the products" and "proceeds to the right").
- Sat Jan 18, 2020 11:58 am
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Defining Le Chatelier's Principle
- Replies: 3
- Views: 248
Re: Defining Le Chatelier's Principle
Le Chatelier's principle states that chemical reactions adjust to minimize the effect of changes.
- Sat Jan 11, 2020 12:03 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Partial Pressure
- Replies: 3
- Views: 206
Re: Partial Pressure
This might be a helpful visual that shows how the total pressure of the gas mixture is the sum of the partial pressures of each individual gas:
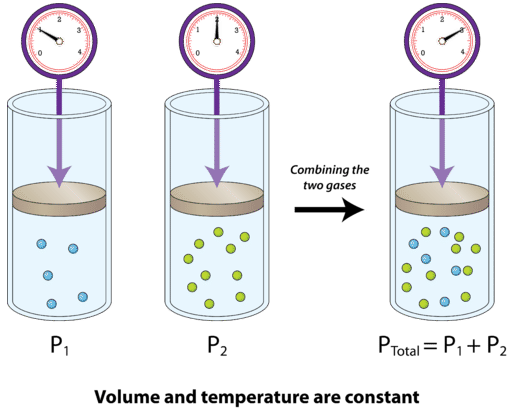

- Sat Jan 11, 2020 11:58 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: ICE Tables
- Replies: 5
- Views: 208
Re: ICE Tables
The activities of pure solids and liquids are equal to 1, which is why they don't affect the equilibrium constant and why you don't include them in your ICE table.
- Sat Jan 11, 2020 11:52 am
- Forum: Ideal Gases
- Topic: Solids and liquids [ENDORSED]
- Replies: 6
- Views: 308
Re: Solids and liquids [ENDORSED]
When trying to understand why liquids and solids are not included in the equilibrium constant, remember that the activity, which Dr. Lavelle discussed when going over equilibrium constants, of any solid or liquid in a reaction is equal to 1.
- Sat Jan 11, 2020 11:39 am
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Le Chatlier Principle
- Replies: 10
- Views: 2118
Re: Le Chatlier Principle
This website has a section called "The Effect of a Catalyst on Equilibrium" that might help you understand more thoroughly what happens if a catalyst is added: https://courses.lumenlearning.com/boundless-chemistry/chapter/factors-that-affect-chemical-equilibrium/
- Sat Jan 11, 2020 11:32 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Significant Figures
- Replies: 8
- Views: 410
Re: Significant Figures
Typically, you see what value in the question has the least amount of significant figures to determine how many significant figures your answer will need to be rounded to, which means you consider both the K constant and the initial concentrations.
- Sat Dec 07, 2019 9:15 am
- Forum: Conjugate Acids & Bases
- Topic: studying
- Replies: 7
- Views: 637
Re: studying
My TA and the UAs that held review sessions recommended knowing the most common strong acids and strong bases.
- Sat Dec 07, 2019 9:13 am
- Forum: Identifying Acidic & Basic Salts
- Topic: Identifying Acidic and Basic Salts
- Replies: 3
- Views: 303
Re: Identifying Acidic and Basic Salts
You identify acidic and basic salts by identifying what kind of ions they have. If a salt contains a cation of a strong base and an anion of a weak acid (like NaOCl), the anion (ClO-) will acts as a base and increase the pH (making the salt basic).
- Sat Dec 07, 2019 9:02 am
- Forum: Lewis Acids & Bases
- Topic: HW 6.5
- Replies: 1
- Views: 226
Re: HW 6.5
A Lewis acid is an electron pair acceptor and a Lewis base is an electron pair donor. From part a of the question, we find that SO3 would react with H2O2 to form H2SO5. Since Sulfur has a vacant orbital, we know that it would accept the electron pair, which makes it the Lewis acid.
- Sat Dec 07, 2019 8:50 am
- Forum: Lewis Acids & Bases
- Topic: HW Problem J.9
- Replies: 1
- Views: 204
Re: HW Problem J.9
For these questions, it is important to know the most common strong acids and bases. When you see the salt (NH4)3PO4, you should recognize it comes from a weak base and strong acid, so when the salt is placed in water it would be acidic (pH<7).
- Sat Dec 07, 2019 8:42 am
- Forum: Properties & Structures of Inorganic & Organic Acids
- Topic: Strong Acids
- Replies: 2
- Views: 332
Re: Strong Acids
In determining the strength of an acid, there are two possible things you look at: 1. Bond Length (the longer the bond, the weaker it is) 2. Stability of the anion (which is the conjugate base)- it can be stabilized by a high electronegativity of one of the atoms and by resonance. You determine whic...
- Thu Nov 28, 2019 9:11 pm
- Forum: Lewis Acids & Bases
- Topic: Lewis acid and base definition
- Replies: 4
- Views: 357
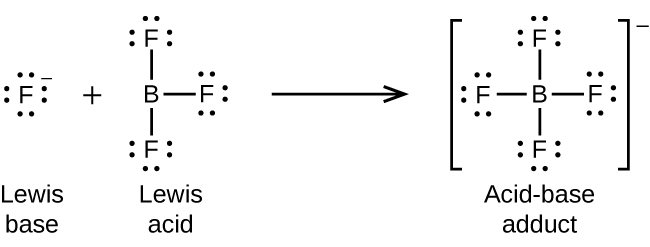
Re: Lewis acid and base definition
Here's an image that depicts the example given about boron trifluoride:

- Thu Nov 28, 2019 9:01 pm
- Forum: Acidity & Basicity Constants and The Conjugate Seesaw
- Topic: Constant K(a)
- Replies: 4
- Views: 281
Re: Constant K(a)
When determining which acid is stronger and the pKa is given, the acid with the lower pKa will be stronger.
- Tue Nov 26, 2019 4:38 pm
- Forum: Naming
- Topic: Polydentate
- Replies: 4
- Views: 200
Re: Polydentate
Here is an example of a polydentate ligand:
Ethylenediaminetetraaceticacid acid (EDTA)
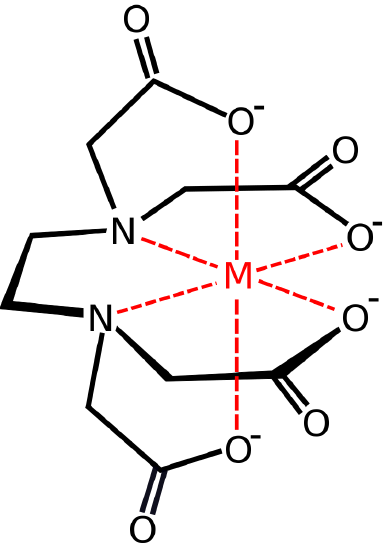
Ethylenediaminetetraaceticacid acid (EDTA)

- Tue Nov 26, 2019 4:22 pm
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: Cis vs Trans
- Replies: 22
- Views: 1756
Re: Cis vs Trans
Here is an example that shows how a cis molecule and a trans molecule look:


- Tue Nov 26, 2019 4:17 pm
- Forum: *Liquid Structure (Viscosity, Surface Tension, Liquid Crystals, Ionic Liquids)
- Topic: Viscosity
- Replies: 25
- Views: 3354
Re: Viscosity
In lecture, we went over the example of 3 hydrocarbons at room temperature: Pentane (C5H12) was a mobile fluid at room temperature. Pentadecane (C15H32) was a viscous fluid at room temperature. Octadecane (C18H35) was a waxy solid at room temperature. In this example, it is clear how the number of e...
- Sun Nov 24, 2019 6:32 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Radicals and Molecular Shape
- Replies: 2
- Views: 216
Re: Radicals and Molecular Shape
The single unpaired electron on a radical is counted as a single region of high electron density, but it has a weaker repulsion than lone pairs. Therefore, the single unpaired electron on a radical does affect the shape of a molecule, but it does not affect the shape of a molecule as much as a lone ...
- Sun Nov 24, 2019 6:18 pm
- Forum: *Molecular Orbital Theory (Bond Order, Diamagnetism, Paramagnetism)
- Topic: Reactivity of pi bonds
- Replies: 2
- Views: 296
Re: Reactivity of pi bonds
A sigma bond by itself is stronger than a pi bond by itself because the atomic orbitals that form sigma bonds overlap more than the orbitals that form pi bonds. Double bonds are stronger than a single bond because they consist of one sigma bond and one pi bond, whereas a single bond consists of only...
- Fri Nov 22, 2019 3:33 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: bent v. angular
- Replies: 27
- Views: 1523
Re: bent v. angular
When the VSEPR formula is AX2E3 or AX2E4, remember that their shapes will be linear (not bent or angular) and they both will have a bond angle of 180 degrees.
- Fri Nov 22, 2019 3:27 pm
- Forum: Polarisability of Anions, The Polarizing Power of Cations
- Topic: polarizability
- Replies: 9
- Views: 824
Re: polarizability
When the intermolecular forces are stronger, it requires more energy to break the bonds, thus making the melting/boiling point higher. For example, CHI3 will have a higher melting point than CHF3 because Iodine has a higher polarizability than Fluorine, which makes the intermolecular forces stronger...
Re: Naming
I think we should know the ones in the table that this link takes you to (the link was included in an email from Dr. Lavelle): https://lavelle.chem.ucla.edu/wp-content/supporting-files/Chem14A/NamingCoordinationCompounds.pdf
- Fri Nov 22, 2019 3:10 pm
- Forum: Lewis Acids & Bases
- Topic: Lewis & Bronsted
- Replies: 3
- Views: 253
Re: Lewis & Bronsted
A Lewis acid is an electron pair acceptor and a Lewis base is an electron pair donor. Whereas, a Bronsted acid is a proton donor and a Bronsted base is a proton acceptor.
- Sat Nov 16, 2019 10:40 am
- Forum: Determining Molecular Shape (VSEPR)
- Topic: test 2
- Replies: 13
- Views: 740
Re: test 2
Debora Fernandez Clemente_ 4H wrote:What are other topics other than the VSEPR models we should know for test two? or is only that?
These are a few of the topics my TA mentioned we should know for Test 2: hydrogen bonding, molecular shape, bond angles, dipole moments, polarity/non-polarity.
- Sat Nov 16, 2019 10:34 am
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Notation used in VSEPR
- Replies: 8
- Views: 587
Re: Notation used in VSEPR
Katherine Brenner 3H wrote:Can someone explain how we get that formula?
For example, NH3 will have a VSEPR formula of AX3E because it has three bonded atoms and one lone pair. CH3Cl will have a VSEPR formula of AX4 because it has four bonded atoms and no lone pairs.
- Sat Nov 16, 2019 10:29 am
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Bond Angle with Lone Pairs
- Replies: 3
- Views: 137
Re: Bond Angle with Lone Pairs
The lone pairs on the central atom influence molecular shape and, thus, affect the bond angles. Specifically, the addition of lone pairs force the bonding electrons closer together, making the bond angles smaller.
- Sat Nov 16, 2019 10:18 am
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Bond Angles
- Replies: 9
- Views: 549
Re: Bond Angles
I believe we should know the bond angles of the molecular shapes we go over during lecture (i.e. Linear-180 degrees & Tetrahedral-109.5 degrees). We predict bond angles when there are lone pairs on the central atom, which influence the molecular shape and affect the bond angle. For example, we c...
- Sat Nov 16, 2019 9:59 am
- Forum: Determining Molecular Shape (VSEPR)
- Topic: How to tell polar or non polar from lewis structure?
- Replies: 9
- Views: 756
Re: How to tell polar or non polar from lewis structure?
Destiny_Ryales_3J wrote:Is this supposed to be on next week’s test?
My TA said that we should know about polar and non-polar molecules for Test 2.
- Sat Nov 09, 2019 6:06 pm
- Forum: Interionic and Intermolecular Forces (Ion-Ion, Ion-Dipole, Dipole-Dipole, Dipole-Induced Dipole, Dispersion/Induced Dipole-Induced Dipole/London Forces, Hydrogen Bonding)
- Topic: Dipole-Dipole vs London
- Replies: 4
- Views: 322
Re: Dipole-Dipole vs London
Examples of Dipole-Dipole Forces:

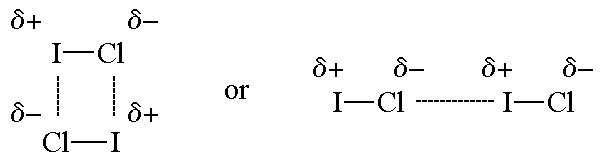

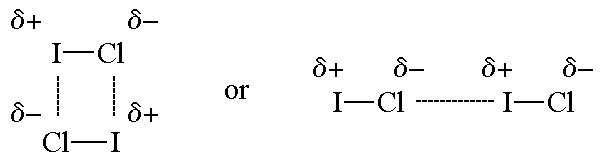
- Sat Nov 09, 2019 5:54 pm
- Forum: Interionic and Intermolecular Forces (Ion-Ion, Ion-Dipole, Dipole-Dipole, Dipole-Induced Dipole, Dispersion/Induced Dipole-Induced Dipole/London Forces, Hydrogen Bonding)
- Topic: Hydrogen Bonding
- Replies: 4
- Views: 261
Re: Hydrogen Bonding
The electronegativity of N, O, and F is important because it affects the strength of the hydrogen bond.
- Sat Nov 09, 2019 5:37 pm
- Forum: Lewis Structures
- Topic: Drawing Lewis Structures
- Replies: 18
- Views: 698
Re: Drawing Lewis Structures
When drawing Lewis structures, keep in mind that you should avoid placing a negative formal charge on the central atom.
- Sat Nov 09, 2019 5:33 pm
- Forum: Lewis Structures
- Topic: Formal Charges
- Replies: 15
- Views: 974
Re: Formal Charges
You generally want to avoid putting a negative formal charge on a central atom.
- Sat Nov 09, 2019 5:22 pm
- Forum: Bond Lengths & Energies
- Topic: Bonds
- Replies: 6
- Views: 431
Re: Bonds
This chart that compares sigma bonds and pi bonds could be helpful to you!


- Sun Nov 03, 2019 1:18 pm
- Forum: Formal Charge and Oxidation Numbers
- Topic: Expanded Octet for Lowest Formal Charge
- Replies: 2
- Views: 118
Re: Expanded Octet for Lowest Formal Charge
Cl can have an expanded octet because it is located in Period 3. Starting from Period 3 (where the principal quantum number is n=3), the d-orbital becomes available and allows the additional electrons to go there, creating an expanded octet.
- Sun Nov 03, 2019 1:10 pm
- Forum: Octet Exceptions
- Topic: What are radicals
- Replies: 7
- Views: 288
Re: What are radicals
A radical is a compound with an unpaired electron, which makes it highly reactive. Radicals are significant because they are exceptions to the octet guideline.
- Sun Nov 03, 2019 1:07 pm
- Forum: Octet Exceptions
- Topic: Expanded Valence Shells
- Replies: 2
- Views: 162
Re: Expanded Valence Shells
Elements starting from period 3 can have an expanded octet because they have a d-orbital that the additional electrons can go into. In other words, the d orbitals become available beginning with the principle quantum number n=3.
- Fri Nov 01, 2019 9:05 pm
- Forum: Trends in The Periodic Table
- Topic: Octet Rules
- Replies: 4
- Views: 200
Re: Octet Rules
If an atom does not satisfy the octet rule, it means there are less than 8 electrons (i.e. the first 4 elements of the periodic table). An expanded octet refers to when there are more than 8 electrons; these are also known as exceptions to the octet rule. Examples of elements that typically have le...
- Fri Nov 01, 2019 9:03 pm
- Forum: Trends in The Periodic Table
- Topic: Octet Rules
- Replies: 4
- Views: 200
Re: Octet Rules
In the review session today, it was mentioned that the elements (with a d-orbital) in Period 3 and later can have an expanded octet.
- Sun Oct 27, 2019 2:52 pm
- Forum: Lewis Structures
- Topic: Bond lengths
- Replies: 3
- Views: 183
Re: Bond lengths
I think the main thing to take away from those values that were given to us is how the single bond length in C—C is longer that the double bond length between 2 Carbons.
- Sun Oct 27, 2019 2:44 pm
- Forum: Trends in The Periodic Table
- Topic: Cations
- Replies: 8
- Views: 371
Re: Cations
On the other hand, anions are bigger than their parent atoms because (as indicated by their negative charge) they gained electrons.
- Sun Oct 27, 2019 2:39 pm
- Forum: Ionic & Covalent Bonds
- Topic: He
- Replies: 3
- Views: 190
Re: He
He has 2 valence electrons, which means it has one electron pair. That is why the Lewis structure for it will look like this- :He
- Sun Oct 27, 2019 2:31 pm
- Forum: Ionic & Covalent Bonds
- Topic: Valence Electrons
- Replies: 4
- Views: 244
Re: Valence Electrons
For example, the electron configuration for Fe is [Ar]4s2 3d6 and we know that it has 2 valence electrons because of 4s2. We do not count the 3d6.
- Sun Oct 27, 2019 2:14 pm
- Forum: *Shrodinger Equation
- Topic: Wave functions
- Replies: 9
- Views: 362
Re: Wave functions
These atomic orbitals are mathematical functions that describe the behavior of the electrons.Sartaj Bal 3H wrote:To express the wave function in three dimensions, the three quantum numbers (n, m, and ml) need to be used. This is known as the atomic orbital, or the region of space where the electron is located.
- Sun Oct 20, 2019 10:24 pm
- Forum: Photoelectric Effect
- Topic: Clarification on Photoelectric Effect
- Replies: 3
- Views: 211
Re: Clarification on Photoelectric Effect
Light sources with long wavelengths (low frequency light) cannot eject electrons even with a high intensity light, which means it does not act like a classical wave. When the frequency increases (or you shorten the wavelength), the energy per photon increases as well, which causes the ejection of el...
- Sun Oct 20, 2019 10:14 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Advice from a Medical Student - Part II [ENDORSED]
- Replies: 298
- Views: 270351
Re: Advice from a Medical Student - Part II [ENDORSED]
Thank you so much for taking the time to share your experience with us and answer our questions! Your advice is extremely helpful and motivating. Is there anything you didn't expect to experience as a medical student that you wish you knew before starting medical school? It wasn't really an experie...
- Sun Oct 20, 2019 10:11 pm
- Forum: Empirical & Molecular Formulas
- Topic: Molecular to Empirical Formula
- Replies: 10
- Views: 990
Re: Molecular to Empirical Formula
You would only be able to do this if the problem provides the molar mass of the molecular formula. This mass is usually provided in problems if they want you to find the molecular formula. You would then divide the given molar mass by the mass of the empirical formula mass. The answer you get is wh...
- Sun Oct 20, 2019 10:08 pm
- Forum: Accuracy, Precision, Mole, Other Definitions
- Topic: States of Matter
- Replies: 9
- Views: 706
Re: States of Matter
It doesn't matter when solving problems for molarity/dilution, but it is is important to include when writing out the chemical equations.
- Sun Oct 20, 2019 10:03 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Accessing the E-textbook [ENDORSED]
- Replies: 125
- Views: 32722
Re: Accessing the E-textbook [ENDORSED]
I don't think it is.Does anyone know if Sapling is mandatory?
- Sat Oct 12, 2019 1:23 pm
- Forum: Significant Figures
- Topic: Significant Figures
- Replies: 6
- Views: 510
Re: Significant Figures
If you want to practice determining how many significant figures a number has, this could be helpful:
https://www.khanacademy.org/math/arithmetic-home/arith-review-decimals/arithmetic-significant-figures-tutorial/e/significant_figures_1
https://www.khanacademy.org/math/arithmetic-home/arith-review-decimals/arithmetic-significant-figures-tutorial/e/significant_figures_1
- Sat Oct 12, 2019 1:21 pm
- Forum: Significant Figures
- Topic: Significant Figures
- Replies: 6
- Views: 510
Re: Significant Figures
Hi! Here are some of the basic rules for significant figures: 1. All non-zero numbers are significant. Ex. 457 has 3 significant figures. 2. Zeros in between two non-zero numbers are significant. Ex. 9023 has 4 significant figures. 3. Trailing zeros (the zeros at the end) are only significant if the...
- Sat Oct 12, 2019 1:02 pm
- Forum: SI Units, Unit Conversions
- Topic: Practice Problems?
- Replies: 11
- Views: 612
Re: Practice Problems?
In our Chemical Principles 7th edition textbook, the practice problems for each of the fundamental sections are located at the end of every section.
- Sat Oct 12, 2019 12:55 pm
- Forum: Molarity, Solutions, Dilutions
- Topic: M1V1=M2V2
- Replies: 11
- Views: 86676
Re: M1V1=M2V2
When I do these type of problems, I often have to convert the units from grams to moles or milliliters to liters. So make sure you pay attention to the units before you start solving the problems using the equations M1V1=M2V2 and Molarity=moles of solute/liters of solution.
- Sat Oct 12, 2019 12:44 pm
- Forum: Molarity, Solutions, Dilutions
- Topic: Dilutions
- Replies: 10
- Views: 1089
Re: Dilutions
When you are solving these type of problems, make sure you pay attention to the units! For this problem, some of the unit conversions you would need to do are converting the grams into moles and milliliters into liters.
- Sat Oct 12, 2019 12:36 pm
- Forum: Significant Figures
- Topic: Balancing Equations
- Replies: 4
- Views: 402
Re: Balancing Equations
When balancing an equation, I first write out how many of each atom are on the side of the reactants and how many of each atom are on the side of the products. If you want to practice balancing chemical equations, this practice on Khan Academy might help you: https://www.khanacademy.org/science/chem...
- Sun Oct 06, 2019 5:58 pm
- Forum: Molarity, Solutions, Dilutions
- Topic: Molar Mass
- Replies: 4
- Views: 182
Re: Molar Mass
Here's an example of how to calculate molar mass: Let's say you need to find the molar mass of NaCl. This can be done by adding the atomic mass of sodium (22.99 g/mol) and the atomic mass of chlorine (35.45 g/mol). (22.99 g/mol) + (35.45g/mol)= 58.44 g/mol Therefore, the molar mass of NaCl will be 5...
- Sun Oct 06, 2019 5:44 pm
- Forum: Accuracy, Precision, Mole, Other Definitions
- Topic: sig figs
- Replies: 20
- Views: 984
Re: sig figs
How do I tell what 0's are significant and which aren't? If you want to practice determining how many significant figures a number has, this could be helpful: https://www.khanacademy.org/math/arithmetic-home/arith-review-decimals/arithmetic-significant-figures-tutorial/e/significant_figures_1
- Sun Oct 06, 2019 5:20 pm
- Forum: Empirical & Molecular Formulas
- Topic: empirical and molecular formulas
- Replies: 7
- Views: 508
Re: empirical and molecular formulas
To see if the empirical formula is the same as the molecular formula or if it is just the ratio, you have to find the molar mass of the empirical formula. If this molar mass is the same as the molar mass of molecular compound, you know that the empirical formula and molecular formula are the same. F...

