Search found 102 matches
- Mon Mar 09, 2020 11:22 am
- Forum: Arrhenius Equation, Activation Energies, Catalysts
- Topic: Catalysts
- Replies: 8
- Views: 532
Re: Catalysts
catalysts provide a different way from going to reactants to products. this way allows for the breaking and forming of bonds to require less energy, because the orientation of the reactants, intermediates and products are different than if the reaction proceeded normally.
- Mon Mar 09, 2020 11:19 am
- Forum: General Rate Laws
- Topic: Overall rate law
- Replies: 8
- Views: 671
Re: Overall rate law
Since this step is the slowest, then the rate of the entire reaction depends on it. Thus, only the concentrations of this reaction intermediate contribute to the overall reaction rate.
- Mon Mar 09, 2020 11:17 am
- Forum: General Rate Laws
- Topic: Enzyme saturation
- Replies: 6
- Views: 428
Re: Enzyme saturation
Once an enzyme becomes saturated, then the reaction is using the enzyme to its best ability (for the amount of reactants, there are enough molecules of enzyme to have the effect on all of it), and so adding more enzyme will not increase the reaction rate .
- Mon Mar 09, 2020 11:16 am
- Forum: Kinetics vs. Thermodynamics Controlling a Reaction
- Topic: Molecularity
- Replies: 12
- Views: 852
Re: Molecularity
Molecularity is how many molecules must collide for product to be formed. It relates to reaction rate because the probability of higher number of molecules all impacting at the same time is lower, and so the reaction rate will naturally be lower.
- Mon Mar 09, 2020 11:14 am
- Forum: Kinetics vs. Thermodynamics Controlling a Reaction
- Topic: Instantaneous Rate
- Replies: 41
- Views: 2416
Re: Instantaneous Rate
The instantaneous rate of change decreases as the reaction proceeds. The reaction gets closer to equilibrium and so the rate decreases.
- Mon Mar 09, 2020 11:13 am
- Forum: Kinetics vs. Thermodynamics Controlling a Reaction
- Topic: reaction rate vs average reaction rate
- Replies: 5
- Views: 552
Re: reaction rate vs average reaction rate
The reaction rate at a certain point is the instantaneous rate of change, or the tangent line to the curve. The average reaction rate is the rate of change over two points.
- Sun Mar 01, 2020 11:43 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Electrolytes vs electrodes
- Replies: 5
- Views: 377
Re: Electrolytes vs electrodes
electrolytes are ions, electrodes connect the two sides of a electrochemical cell in order to allow the flow of electrons
- Sun Mar 01, 2020 11:40 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Half reactions and cell potential
- Replies: 2
- Views: 199
Re: Half reactions and cell potential
the table lists the voltage for the standard reduction potentials. however, if in a reaction that substance is undergoing oxidation, you need to flip the equation and thus change the sign of the voltage.
- Sun Mar 01, 2020 11:36 pm
- Forum: Work, Gibbs Free Energy, Cell (Redox) Potentials
- Topic: reaction supported through kinetics
- Replies: 2
- Views: 278
Re: reaction supported through kinetics
he stated this because although the reaction is thermodynamically favored, the activation energy is high enough to stop the reaction from happening spontaneously.
- Sun Mar 01, 2020 11:34 pm
- Forum: Work, Gibbs Free Energy, Cell (Redox) Potentials
- Topic: n value
- Replies: 9
- Views: 613
Re: n value
The n value is the number of electrons that are transferred in a reaction.
- Sun Mar 01, 2020 11:34 pm
- Forum: Work, Gibbs Free Energy, Cell (Redox) Potentials
- Topic: n value
- Replies: 9
- Views: 613
Re: n value
The n value is the number of electrons that are transferred in a reaction.
- Sun Mar 01, 2020 11:33 pm
- Forum: Work, Gibbs Free Energy, Cell (Redox) Potentials
- Topic: Voltage vs Gibbs
- Replies: 2
- Views: 234
Voltage vs Gibbs
Why is voltage positive when the reaction is spontaneous while the gibbs free energy is negative?
- Sun Feb 23, 2020 8:15 pm
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: Standard vs Non
- Replies: 3
- Views: 268
Re: Standard vs Non
Standard cell potentials are the potentials of cells at standard conditions (298.15 K, 1 atm, etc.) while non-standard are the cell potentials at any other set of conditions.
- Sun Feb 23, 2020 8:13 pm
- Forum: Work, Gibbs Free Energy, Cell (Redox) Potentials
- Topic: Spontaneity
- Replies: 7
- Views: 602
Re: Spontaneity
Cell potential has nothing to do with reaction speed, although there are other factors that can speed up a reaction with a large cell potential. Think of it as the distance in energy between the products and reactants.
- Sun Feb 23, 2020 8:10 pm
- Forum: Balancing Redox Reactions
- Topic: Flow of electrons
- Replies: 11
- Views: 828
Re: Flow of electrons
There are some confused answers here. The anode is not necessarily always negative — neither is the cathode always positive (exceptions are in non-spontaneous reactions). However, the anode is always oxidation and the cathode is always reduction, so then the electrons flow from anode to cathode.
- Sun Feb 23, 2020 8:02 pm
- Forum: Balancing Redox Reactions
- Topic: Steps
- Replies: 7
- Views: 432
Re: Steps
It's not quite the same. The first part is different. First, you want to make sure that the half reactions have balanced electrons and then once that is done, you can balance the rest of the reaction normally.
- Sun Feb 23, 2020 8:00 pm
- Forum: Balancing Redox Reactions
- Topic: Half Reactions
- Replies: 12
- Views: 889
Re: Half Reactions
A half reaction is a way of representing a certain part of a redox reaction. It shows just the process of either reduction or oxidation and the electrons that are involved.
- Sun Feb 16, 2020 8:44 pm
- Forum: Van't Hoff Equation
- Topic: When to apply the Van't Hoff Equation
- Replies: 5
- Views: 753
Re: When to apply the Van't Hoff Equation
The vant hoff equation is used when we want to calculate the K of a reaction at a certain temperature. Gibbs free energy is mostly to determine if a reaction will be spontaneous at a given temperature.
- Sun Feb 16, 2020 8:40 pm
- Forum: Van't Hoff Equation
- Topic: Equilibrium
- Replies: 6
- Views: 769
Re: Equilibrium
It relates the equilibrium constant, which shows the relative concentrations of each substance at a certain temperature, to the temperature, delta S, and delta H.
- Sun Feb 16, 2020 8:37 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Midterm Curve
- Replies: 45
- Views: 2388
Re: Midterm Curve
The cutoffs for final grades are curved but we don't exactly know what the cutoffs are.
- Sun Feb 16, 2020 8:36 pm
- Forum: Van't Hoff Equation
- Topic: Van Hoff's Constants
- Replies: 6
- Views: 355
Re: Van Hoff's Constants
Delta H and delta S are constant. Even though temperature might change the absolute entropy or enthalpy of a system. the change in that value over a reaction will remain constant over different temperatures.
- Sun Feb 16, 2020 8:34 pm
- Forum: Phase Changes & Related Calculations
- Topic: change in entropy
- Replies: 7
- Views: 688
Re: change in entropy
delta S means the change in the disorder of a system. If a system gets more ordered, then the entropy change is positive
- Sun Feb 16, 2020 8:32 pm
- Forum: Kinetics vs. Thermodynamics Controlling a Reaction
- Topic: 6K3
- Replies: 3
- Views: 397
6K3
How do I balance this using half reactions?
(a) Reaction of thiosulfate ion with chlorine gas:
Cl2(g) + S2O32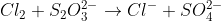
(aq) -> Cl2(aq) 1 SO422 (aq)
(a) Reaction of thiosulfate ion with chlorine gas:
Cl2(g) + S2O32
(aq) -> Cl2(aq) 1 SO422 (aq)
- Sun Feb 16, 2020 8:25 pm
- Forum: Kinetics vs. Thermodynamics Controlling a Reaction
- Topic: 6K
- Replies: 4
- Views: 459
6K
The following redox reaction is used in acidic solution in the Breathalyzer test to determine the level of alcohol in blood: H1 (aq) + Cr2O722 (aq) + C2H5OH(aq) -> Cr31 (aq) + C2H4O(aq) + H2O(l) Identify the elements undergoing oxidation or reduction and indicate their initial and final oxidation nu...
- Sun Feb 09, 2020 7:05 pm
- Forum: Entropy Changes Due to Changes in Volume and Temperature
- Topic: Predicting Entropy
- Replies: 2
- Views: 232
Re: Predicting Entropy
Entropy is best determined by the amount of substance, the phase of the substance, and the complexity of the molecule.
- Sun Feb 09, 2020 7:01 pm
- Forum: Phase Changes & Related Calculations
- Topic: Enthalpy of a combustion reaction?
- Replies: 7
- Views: 479
Re: Enthalpy of a combustion reaction?
delta H is negative. Heat is being released as the products are entering a more stable state as they form reactants.
- Sun Feb 09, 2020 6:59 pm
- Forum: Phase Changes & Related Calculations
- Topic: -w vs w
- Replies: 15
- Views: 701
Re: -w vs w
w is a change in the internal energy of a system that is caused by a physical change (as opposed to Q which is the heat). if w is positive, then energy is being put into the system (work is being done on the system.
- Sun Feb 09, 2020 6:58 pm
- Forum: Phase Changes & Related Calculations
- Topic: Heating Curve Phase Changes
- Replies: 11
- Views: 637
Re: Heating Curve Phase Changes
During the flat part of a phase diagram, all the heat is being used to separate the molecules and thus change the phase. There is no heat being contributed to the random motion of the particles and thus the temperature remains constant.
- Sun Feb 09, 2020 6:55 pm
- Forum: Phase Changes & Related Calculations
- Topic: Heat & Temperature of Phase Changes
- Replies: 5
- Views: 154
Re: Heat & Temperature of Phase Changes
There is no set rate of how much heat is being used to change phase. There is a certain amount of heat that is required to change phases, and once that amount of heat is inputted into the substance, it changes phase and any more heat added contributes to the temperature.
- Sat Feb 01, 2020 11:08 pm
- Forum: Thermodynamic Systems (Open, Closed, Isolated)
- Topic: Pressure in an open beaker
- Replies: 12
- Views: 824
Re: Pressure in an open beaker
Adding moles of gas to the atmosphere does not change its overall pressure in a way that's measurable. Therefore, an open system has a constant external pressure.
- Sat Feb 01, 2020 11:04 pm
- Forum: Thermodynamic Systems (Open, Closed, Isolated)
- Topic: Isolated vs Closed [ENDORSED]
- Replies: 34
- Views: 4466
Re: Isolated vs Closed [ENDORSED]
By strict definition, there is no such thing as an isolated system. So, things that come close to being isolated count as isolated—a thermos would.
- Sat Feb 01, 2020 11:02 pm
- Forum: Calculating Work of Expansion
- Topic: ∆H and ∆U
- Replies: 3
- Views: 193
Re: ∆H and ∆U
Delta H is the change in enthalpy of a system while delta U is the change in internal energy accompanied by a reaction. In other words, the difference between delta H and delta U is P x Delta V. So Delta H is the total work done and Delta U is just the work done by the heat of the reaction.
- Sat Feb 01, 2020 10:41 pm
- Forum: Thermodynamic Definitions (isochoric/isometric, isothermal, isobaric)
- Topic: reversible vs irreversible expansion
- Replies: 4
- Views: 186
Re: reversible vs irreversible expansion
In the reversible case, the external pressure is slowly decreased, where there is sort of a quasi equilibrium happening constantly and thus the internal gas does more work to push out. If you decrease the external pressure instantly and dramatically, the internal gas pushed out quickly and does less...
- Sat Feb 01, 2020 10:20 pm
- Forum: Thermodynamic Systems (Open, Closed, Isolated)
- Topic: isolated system
- Replies: 13
- Views: 646
Re: isolated system
a very well designed thermos that allows negligible heat transfer
- Thu Jan 23, 2020 8:19 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Heat Capacity Intensive or Extensive?
- Replies: 4
- Views: 680
Re: Heat Capacity Intensive or Extensive?
Depends on if you're talking about specific heat capacity or just heat capacity. Heat capacity is how much energy is required to increase the temperature of an arbitrary amount of substance—if the amount is increased, the heat capacity is increased. However, specific heat capacity is how much energy...
- Thu Jan 23, 2020 8:13 pm
- Forum: Phase Changes & Related Calculations
- Topic: Autoprotolysis
- Replies: 15
- Views: 867
Re: Autoprotolysis
Protolysis is a process that occurs with polyprotic substances. There are occurrences where a molecule can give another of the same type of molecule a proton.
- Thu Jan 23, 2020 8:08 pm
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: 5.61f
- Replies: 5
- Views: 210
Re: 5.61f
Yes. If they were all aqueous, then changing the amount of H2O would change the concentrations, and cause a shift. This is a similar principle to gases in a vessel that are being compressed and expanded.
- Thu Jan 23, 2020 8:06 pm
- Forum: Phase Changes & Related Calculations
- Topic: Enthalpy Units
- Replies: 3
- Views: 202
Re: Enthalpy Units
The delta H of an equation is actually measured in energy per unit substance—usually kJ/mol or J/mol.
- Thu Jan 23, 2020 8:02 pm
- Forum: Phase Changes & Related Calculations
- Topic: Steam vs liquid
- Replies: 7
- Views: 376
Re: Steam vs liquid
Although steam and water may both be at 100 degrees C, the steam will give you more of a burn. Both steam and water will want to reach an equilibrium with whatever is being burned, eventually reaching 25 degrees. However, the energy given off by steam on its way to this temperature is much greater t...
- Thu Jan 23, 2020 7:57 pm
- Forum: Phase Changes & Related Calculations
- Topic: State Property
- Replies: 6
- Views: 394
Re: State Property
A state property is a property that depends on only the two states, initial and final, of the substance being measured. The example that Lavelle used was potential energy, where the height of the hikers on the mountain determined their potential energy, regardless of the path that was taken to get t...
- Sat Jan 18, 2020 5:59 pm
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: 5J.1 a)
- Replies: 5
- Views: 146
Re: 5J.1 a)
This decreases the partial pressure of H2 as to shift the reaction in the direction of the reactants, both products are needed (Products can only be formed with both CO2 and H2).
- Sat Jan 18, 2020 5:55 pm
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: 5 %
- Replies: 4
- Views: 263
5 %
Why is the 5% rule a thing? It seems a bit arbitrary to neglect that much of a change in the amount of reactants. Is there any practical basis in the rule.
- Sat Jan 18, 2020 5:54 pm
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Change in Pressure
- Replies: 5
- Views: 187
Re: Change in Pressure
There is a natural point where there will the energy will be the lowest for a certain reaction. Some reactions will be at their lowest energy when there are a relatively high number of products, and vice versa. Thus, when there is a change in the number of moles of a reaction, then that reaction wil...
- Sat Jan 18, 2020 5:50 pm
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Pressure
- Replies: 3
- Views: 138
Re: Pressure
A change in volume will shift the equilibrium of a mixture. However, the change in pressure is not so cut and dry. If one changes the pressure by changing the volume, then there will be a shift in equilibrium. However, if one changes the pressure of the entire vessel by adding more of another gas. T...
- Sat Jan 18, 2020 5:47 pm
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Kb vs Ka
- Replies: 5
- Views: 168
Kb vs Ka
I understand how in lecture Mr Lavelle told us that the Kb of a weak acid was Kw over the Ka. But does this represent the Kb of that same compound, or the Kb of its conjugate base? For example, would the Kb calculated from the Ka of HSO4 be the Ka of HSO4 or the Ka of H2SO4?
- Sat Jan 11, 2020 3:58 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: K vs. Q
- Replies: 10
- Views: 559
Re: K vs. Q
K is the experimentally determined value of the ratio of products to reactants after the reaction has reached equilibrium. Q is the current ratio of products to reactants in a reaction.
- Sat Jan 11, 2020 3:56 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Partial Pressure
- Replies: 7
- Views: 345
Re: Partial Pressure
Partial pressure is a measurement of the pressure on a vessel contributed by a certain substance. This is useful when there are multiple gases in the same vessel, where you need to distinguish total pressure from the partial pressure of each substance.
- Sat Jan 11, 2020 3:50 pm
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: 5.61b
- Replies: 3
- Views: 203
Re: 5.61b
On both sides of the reaction, there are 6 moles of gas, so changing the pressure will not affect the equilibrium.
- Sat Jan 11, 2020 3:48 pm
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Decreasing pressure by increasing volume [ENDORSED]
- Replies: 4
- Views: 173
Re: Decreasing pressure by increasing volume [ENDORSED]
There will be the same effect on the equilibrium in the opposite direction. Decreasing the pressure (by increasing the volume) will push the equilibrium in the direction of the side with more moles of gas.
- Sat Jan 11, 2020 3:47 pm
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: HW 5J.5
- Replies: 8
- Views: 216
Re: HW 5J.5
The left side of the equation will be favored because it has less total moles of gas (Carbon is solid).
- Mon Dec 02, 2019 9:30 am
- Forum: Properties & Structures of Inorganic & Organic Acids
- Topic: Electronegativity and acid strength
- Replies: 2
- Views: 139
Electronegativity and acid strength
Why would an acid such as HClO be stronger than, say, HBrO?
- Mon Dec 02, 2019 9:30 am
- Forum: Properties & Structures of Inorganic & Organic Acids
- Topic: Electronegativity and acid strength
- Replies: 5
- Views: 301
Electronegativity and acid strength
Why would an acid such as HClO be stronger than, say, HBrO?
- Mon Dec 02, 2019 9:22 am
- Forum: Amphoteric Compounds
- Topic: Acidic vs Basic oxides
- Replies: 2
- Views: 162
Re: Acidic vs Basic oxides
Not all oxides are amphoteric. Only oxides involving the elements Be, Al, Ga, Ge, As, In, SN, Sb, Pb, and Bi (as shown in the textbook diagram) are amphoteric. What you need to know for sure is that metal oxides form bases and non-metal oxides form acids. The reactions that prove these two facts are...
- Mon Dec 02, 2019 9:16 am
- Forum: Calculating pH or pOH for Strong & Weak Acids & Bases
- Topic: pH scale
- Replies: 12
- Views: 692
Re: pH scale
A scale from 1-14 is most common as others have said and it encompasses all solutions found in organic systems.
- Mon Dec 02, 2019 9:15 am
- Forum: Naming
- Topic: Coordination Compound: Cation or Anion
- Replies: 3
- Views: 353
Re: Coordination Compound: Cation or Anion
This is the norm with ionic compounds (cations written before anions). I can't say if your answer will be marked wrong if you put it the other way, but it isn't hard to put the cation before the anion (if you don't know the charge on the complex ion, just use the charge on the other ion and work fro...
- Mon Dec 02, 2019 9:12 am
- Forum: Naming
- Topic: Neutral Molecules
- Replies: 2
- Views: 256
Re: Neutral Molecules
I have found this trend too. But I have found some questions involving ethylenediamine. I wouldn't be surprised if he asked some questions involving this ligand (represented as en) or diethylenetriamine (dien) to test our knowledge of chelates.
- Tue Nov 26, 2019 4:58 pm
- Forum: Biological Examples
- Topic: EDTA
- Replies: 8
- Views: 593
EDTA
What is EDTA and how does it remove heavy metals from the blood?
- Tue Nov 26, 2019 4:55 pm
- Forum: Naming
- Topic: Organometallic complex
- Replies: 2
- Views: 149
Organometallic complex
What's the difference between a organometallic complex and a coordination compound?
- Tue Nov 26, 2019 4:53 pm
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: Coordination Number
- Replies: 3
- Views: 249
Re: Coordination Number
The coordination number is the number of ligands that are bound to the central atoms. This determines the shape of the molecule and the shape of the molecule determines how it interacts with other molecules.
- Tue Nov 26, 2019 4:47 pm
- Forum: Conjugate Acids & Bases
- Topic: Cojugate Acids and Bases
- Replies: 10
- Views: 2253
Re: Cojugate Acids and Bases
The conjugate acid and conjugate base are only used when talking about a molecule in reference to another. A conjugate acid of something will have one more H than the thing it's a conjugate of. A conjugate base will have one less H than the the thing it's a conjugate of.
- Tue Nov 26, 2019 4:44 pm
- Forum: Bronsted Acids & Bases
- Topic: Bronsted Acids and Bases
- Replies: 6
- Views: 439
Re: Bronsted Acids and Bases
Understand that bronsted acids are molecules that donate an H+ ion and bronsted bases are those that receive an H+ ion.
- Sun Nov 24, 2019 1:39 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Bond Angle of bent
- Replies: 17
- Views: 1409
Re: Bond Angle of bent
This depends on the vsepr formula of the molecule. there can be a bent shape in both AX2E2 and AX2E but the angles will be different in these two cases. In AX2E2 there will be more electron repulsion (its electron geometry is tetrahedral) so it will be slightly less than 109.5. In AX2E, the electron...
- Sun Nov 24, 2019 1:34 pm
- Forum: Naming
- Topic: Heme Complex
- Replies: 3
- Views: 252
Re: Heme Complex
The heme complex is an iron molecule bound to a porphyrin ligand. The porphyrin ligand is a tetradentate ring structure, meaning the fact that there are four nitrogens facing inward that can can form coordination complexes with the iron.
- Sun Nov 24, 2019 1:29 pm
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: Chelating Ligands
- Replies: 3
- Views: 207
Re: Chelating Ligands
Chelating ligands are flexible molecules that have sigma bonds and can form multiple coordination compounds with the same transition metal. This makes them especially strong.
- Sun Nov 24, 2019 1:26 pm
- Forum: Dipole Moments
- Topic: Dipole Induced- Dipole Induced
- Replies: 13
- Views: 1214
Re: Dipole Induced- Dipole Induced
Yes, dipole induced-dipole induced is the same as Van Der Waals and London dispersion forces.
- Sun Nov 24, 2019 1:24 pm
- Forum: Biological Examples
- Topic: Transplatin?
- Replies: 3
- Views: 250
Re: Transplatin?
transplatin IS able to form coordination complex with DNA, but only one. The middle bond on the cisplatin and transplatin molecule prevents rotation in the molecule so transplatin can only form a coordination complex on one side, but the other side is facing the other way and can't rotate to form a ...
- Tue Nov 12, 2019 10:04 am
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Forces
- Replies: 3
- Views: 114
Forces
I heard that molecules with more lone pairs on each atom will have a longer bond length. Why?
- Tue Nov 12, 2019 10:02 am
- Forum: Determining Molecular Shape (VSEPR)
- Topic: VSEPR
- Replies: 5
- Views: 147
VSEPR
I heard from my TA this thing called VSEPR. I heard it was about naming shapes of molecules. Are we supposed to memorize all the names of the molecular shapes?
- Tue Nov 12, 2019 9:57 am
- Forum: Dipole Moments
- Topic: 3f.1
- Replies: 3
- Views: 214
Re: 3f.1
NH2OH is a molecule that has a very weak dipole moment (the electrons are distributed unevenly throughout the molecule, but not that unevenly). Thus, there will be dipole-dipole and london dispersion. Also, after drawing the Lewis structure, you will see that hydrogen has an N on one side and an O o...
- Tue Nov 12, 2019 9:36 am
- Forum: Dipole Moments
- Topic: 3f.15
- Replies: 3
- Views: 276
Re: 3f.15
Both of the above molecules are non-polar so the intermolecular forces in both cases will be induced dipole-induced dipole. The larger molecule will have a stronger force of this kind as their electrons can be distorted more. Thus, AsF5 will have a higher boiling point as it takes more energy to mov...
- Tue Nov 12, 2019 9:30 am
- Forum: Formal Charge and Oxidation Numbers
- Topic: Formal Charge Placement
- Replies: 4
- Views: 516
Re: Formal Charge Placement
The positive charge will depend on where the negative charges are. Octet rules are obeyed first, then electronegativity and lowest formal charge should be obeyed, and then the positive charges will be in the places that they should be. It's not a matter of placing the positive charges where they sho...
- Mon Nov 04, 2019 9:49 am
- Forum: Ionic & Covalent Bonds
- Topic: Ionic and covalent bonds
- Replies: 2
- Views: 120
Re: Ionic and covalent bonds
when drawing ionic compounds using lewis dot diagrams, have both separate ions as lewis structures in their own set of brackets beside each other. put the charge on that ion in the top right of the brackets.
- Mon Nov 04, 2019 9:43 am
- Forum: Lewis Structures
- Topic: Oxygen
- Replies: 2
- Views: 233
Re: Oxygen
This is more important when we get into VSEPR models and have to obey a law that electron pairs want to be as far away from each other as possible. not sure if drawing diagonally in lewis structures is necessary for the test, but it would be a good habit to get into regardless and is more correct.
- Mon Nov 04, 2019 9:39 am
- Forum: Octet Exceptions
- Topic: Octet Exceptions
- Replies: 2
- Views: 140
Re: Octet Exceptions
Elements that can have expanded octets that we need to know will be in the third group, as they have a 3d subshell (though usually empty) that can be filled to accommodate extra valence electrons.
- Mon Nov 04, 2019 9:33 am
- Forum: Trends in The Periodic Table
- Topic: Electronegativity
- Replies: 11
- Views: 759
Re: Electronegativity
There will be a lot of drawing of lewis structures. a good understanding of electronegativity is essential to drawing the correct lewis structures so it would be good to brush up on it.
- Mon Nov 04, 2019 9:32 am
- Forum: Electronegativity
- Topic: Difference
- Replies: 5
- Views: 324
Re: Difference
electronegativity is the tendency of an atom within a chemical bond to attract electrons to itself. this is why when the difference in electronegativity is high enough, one atom rips an electron off of another, as this atom has a tendency to pull electrons to itself so much more than the other. pola...
- Mon Oct 28, 2019 9:44 am
- Forum: Quantum Numbers and The H-Atom
- Topic: H atom
- Replies: 3
- Views: 154
Re: H atom
To make this simpler, one can just look at the ionization energy of the H atom. The H atom has one electron, and so calculating the ionization energy (using coulomb's law) is much much simpler than calculating the ionization energies of atoms with multielectron systems. Although we are not performin...
- Mon Oct 28, 2019 9:40 am
- Forum: Electron Configurations for Multi-Electron Atoms
- Topic: 1E.25
- Replies: 1
- Views: 174
Re: 1E.25
a) Alkali metals all have one electron in their outer shell (which is why they're so reactive) and so its outer shell can be represented as ns1. b) group 15 elements have 3 electrons in their outermost p orbital and a full s orbital. So then the outer shell can be represented by ns2 np3 c) group 5 t...
- Mon Oct 28, 2019 9:33 am
- Forum: Ionic & Covalent Bonds
- Topic: 2A.11
- Replies: 4
- Views: 238
Re: 2A.11
To find the 3+ ions of the specified electron configurations you need to consider that metals lose their s electrons first. So in the case of all these metals you would just be moving one to the right on the periodic table as the other two electrons are lost from the s orbital of the shell above.
- Mon Oct 28, 2019 9:14 am
- Forum: Ionic & Covalent Bonds
- Topic: Anions and Cations
- Replies: 4
- Views: 258
Re: Anions and Cations
The tendency of an atom to make cations or anions depends on their ionization energy. Atoms to the left of the metalloids usually form cations, and those to the left usually form anions. The metalloids are the separating factor as they are the atoms for which the ionization energy is intermediate, m...
- Mon Oct 28, 2019 9:06 am
- Forum: Electron Configurations for Multi-Electron Atoms
- Topic: HW question 1D.11
- Replies: 5
- Views: 201
Re: HW question 1D.11
a) the subshell with l = 0 is the s subshell and so it will have one orbital b) the subshell with l = 2 is the d subshell and so it will have 5 orbitals c) the subshell with l = 1 is the s subshell and so it will have 3 orbitals d) the subshell with l = 3 is the f subshell and so it will have 7 orbi...
- Mon Oct 21, 2019 9:22 am
- Forum: Trends in The Periodic Table
- Topic: 1F.6
- Replies: 1
- Views: 98
Re: 1F.6
It is easier to do this when you have a periodic table. Just subtract one electron and move to the left of the element in question to see how many electrons the element in question will have. For example S+ will have the same electron configuration as P. Do this again for the second ionization energ...
- Mon Oct 21, 2019 9:18 am
- Forum: General Science Questions
- Topic: Quick clarification
- Replies: 4
- Views: 326
Re: Quick clarification
Planck's constant is useful for calculations involving things at the quantum scale and, as others have stated above, is used in equations like E = hv etc.
- Mon Oct 21, 2019 9:16 am
- Forum: Trends in The Periodic Table
- Topic: Difference between electron affinity and electronegativity?
- Replies: 3
- Views: 157
Re: Difference between electron affinity and electronegativity?
electron affinity is the ionization energy of the negative ion of an element. it's measured by giving an electron to said atom and then measuring how much energy is required to take it off. sometimes, this energy is positive, as in the case of noble gases, as the atom being so stable does not want t...
- Mon Oct 21, 2019 9:11 am
- Forum: Electron Configurations for Multi-Electron Atoms
- Topic: Exceptions in Electron Configurations
- Replies: 5
- Views: 211
Re: Exceptions in Electron Configurations
In the case of Cr and Mo, the lower energy state is achieved by moving an electron from the higher energy 4s to the lower energy 3d to have a "full" half-shell meaning that all the electrons within 3d have the same spin. In Cu, Ag, Au, the electron from the higher energy 4s moves to fill t...
- Mon Oct 21, 2019 9:07 am
- Forum: Wave Functions and s-, p-, d-, f- Orbitals
- Topic: terminology - "orbitals", "shells", "subshells"
- Replies: 2
- Views: 142
Re: terminology - "orbitals", "shells", "subshells"
One can say that there exists a "4p orbital" meaning an orbital that exists within the 4p subshell but "4p" isn't an orbital itself. The magnetic quantum number refers to the specific orbital within said subshell
- Tue Oct 15, 2019 10:30 pm
- Forum: Properties of Light
- Topic: Variables and what they mean
- Replies: 9
- Views: 541
Re: Variables and what they mean
lambda is the wavelength of a wave. v is the frequency of a wave.
- Tue Oct 15, 2019 10:26 pm
- Forum: Bohr Frequency Condition, H-Atom , Atomic Spectroscopy
- Topic: Atomic Spectra Wave Model or Particle Model?
- Replies: 3
- Views: 130
Re: Atomic Spectra Wave Model or Particle Model?
Both. The reason the electrons are quantized in their orbits around the nucleus is because of their wavelike properties (remember how in lecture, Lavelle showed how if the electron was in the wrong position, it would be cancelled out by its own wave). However, as an electron "drops" from a...
- Tue Oct 15, 2019 10:18 pm
- Forum: Heisenberg Indeterminacy (Uncertainty) Equation
- Topic: Building arguments on indeterminacy equation
- Replies: 2
- Views: 106
Building arguments on indeterminacy equation
How can we base our knowledge on the nature of electrons off of a faulty measurement? Just because we can't measure something, it doesn't necessarily mean it's in a cloud of probable locations.
- Tue Oct 15, 2019 10:15 pm
- Forum: SI Units, Unit Conversions
- Topic: Joules
- Replies: 2
- Views: 166
Joules
What are the units for joules? I remember it was something complicated and I forgot what. Why are they that way?
- Tue Oct 15, 2019 1:36 pm
- Forum: Heisenberg Indeterminacy (Uncertainty) Equation
- Topic: Indeterminacy Equation
- Replies: 5
- Views: 231
Re: Indeterminacy Equation
The equation uses delta p to refer to the range that we are uncertain about. The object in question will then have a momentum at any instant within that range of delta p.
- Mon Oct 07, 2019 11:34 am
- Forum: Properties of Light
- Topic: Light and our Skin
- Replies: 2
- Views: 252
Light and our Skin
Why would UV radiation be harmful to our skin in terms of threshold energy?
- Mon Oct 07, 2019 11:31 am
- Forum: SI Units, Unit Conversions
- Topic: Intensive Properties
- Replies: 3
- Views: 109
Intensive Properties
If density is an intensive property and does not rely on the amount of substance, why does the density of gas change as its amount changes?
- Mon Oct 07, 2019 11:28 am
- Forum: Properties of Light
- Topic: Spectroscopy
- Replies: 2
- Views: 80
Spectroscopy
Why is spectroscopy useful in a practical sense?
- Mon Oct 07, 2019 11:27 am
- Forum: Properties of Light
- Topic: Work Function
- Replies: 4
- Views: 156
Work Function
What is the difference between the work function and the threshold energy?
- Mon Oct 07, 2019 11:22 am
- Forum: Properties of Light
- Topic: 1A- Electromagnetic Radiation
- Replies: 4
- Views: 240
Re: 1A- Electromagnetic Radiation
We can find the answer through elimination. a) is incorrect because c, the speed of light is a constant. b) is incorrect because the wavelength would increase. A decrease in frequency would mean an increase in wavelength because v = c/wavelength. d) is incorrect because the energy would definitely n...
- Sun Sep 29, 2019 1:34 pm
- Forum: Empirical & Molecular Formulas
- Topic: Empirical Formula
- Replies: 6
- Views: 171
Empirical Formula
When is it important to use the empirical formula rather than the molecular formula?
- Sun Sep 29, 2019 1:33 pm
- Forum: Balancing Chemical Reactions
- Topic: Balancing equations
- Replies: 7
- Views: 6097
Balancing equations
Is it okay to have decimals in a balanced chemical equation? Why or why not?
- Sun Sep 29, 2019 1:29 pm
- Forum: Significant Figures
- Topic: Basic sig fig question
- Replies: 6
- Views: 532
Re: Basic sig fig question
Also there are simply cases where doing a list of operations in different orders (ie. addition before multiplication or vice versa) will yield different numbers of sig figs.
- Sun Sep 29, 2019 1:28 pm
- Forum: Significant Figures
- Topic: Basic sig fig question
- Replies: 6
- Views: 532
Re: Basic sig fig question
Some steps that involve addition and subtraction will require the use of different sig fig rules. As long as you are careful to pay attention to these operations then it is better to keep more sig figs than needed up until the final calculation to get a more accurate result.

