Search found 102 matches
- Sun Mar 15, 2020 7:42 am
- Forum: Ideal Gases
- Topic: Kc vs Kp
- Replies: 109
- Views: 4986
Re: Kc vs Kp
Kc is used if you are given concentrations and Kp is used when you are given partial pressures.
- Sun Mar 15, 2020 7:40 am
- Forum: Entropy Changes Due to Changes in Volume and Temperature
- Topic: Difference in volume and temperature
- Replies: 6
- Views: 572
Re: Difference in volume and temperature
for which equation?
- Sun Mar 15, 2020 7:39 am
- Forum: Zero Order Reactions
- Topic: 0 order
- Replies: 7
- Views: 691
Re: 0 order
If the graph of [A] v. time is a negative slope straight line. Other than that you find the order of a reaction through experimental data. Zero orders do not affect the reaction rate and therefore Rate=k.
- Sun Mar 15, 2020 7:37 am
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: platinum
- Replies: 4
- Views: 374
Re: platinum
Its the side whose reaction does not already involve a solid metal and therefore platinum is added as an electrode to conduct the reaction.
- Sun Mar 15, 2020 7:36 am
- Forum: Administrative Questions and Class Announcements
- Topic: Constants and Equations
- Replies: 1
- Views: 198
Constants and Equations
For finding standard Gibbs free energy formations and standard reduction reaction values, would we just have to use the textbook? The constant and equation sheet does not have the same amount of resources than the one we received on the midterm.
- Sun Mar 08, 2020 11:09 pm
- Forum: General Rate Laws
- Topic: slow step
- Replies: 9
- Views: 650
Re: slow step
Its the rate determining step unless it does not match up with the actual rate law of the reaction.
- Sun Mar 08, 2020 11:01 pm
- Forum: Balancing Redox Reactions
- Topic: Why do we flip E for oxidation?
- Replies: 13
- Views: 1167
Re: Why do we flip E for oxidation?
You do not flip the sign if you are calculating the standard cell potential for the anode value.
- Sun Mar 08, 2020 10:45 pm
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: Purpose of Nernst Equation
- Replies: 5
- Views: 416
Re: Purpose of Nernst Equation
The nernst equation is used to calculate the cell potential anytime the cell is not in standard conditions. It can be applied to galvanic cells, concentration cells, electrolytic cells. As long as the number of electrons transferred and the standard cell potential is known, it can be used.
- Sun Mar 08, 2020 10:41 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: test 1
- Replies: 2
- Views: 221
Re: test 1
You would get the H+ concentration from the pH and then use that as the change in your ice table. The problem gives you the initial concentration of the acid and if you subtract that from the H+ concentration you get the equilibrium concentration for HF. Input these values into the Ka equilibrium eq...
- Sun Mar 08, 2020 10:22 pm
- Forum: General Rate Laws
- Topic: arrhennius question
- Replies: 4
- Views: 307
Re: arrhennius question
Any problem that mentions trying to find the activation energy.
- Sun Mar 01, 2020 10:54 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Anode and Cathode
- Replies: 8
- Views: 596
Re: Anode and Cathode
Anode is where the oxidation half reaction of the redox reaction takes place and cathode is where the reduction half reaction takes place. Since the oxidation in the solution of the anode is losing electrons, these electrons travel through the anode which is a solid metal to the cathode another soli...
- Sun Mar 01, 2020 10:51 pm
- Forum: Interesting Applications: Rechargeable Batteries (Cell Phones, Notebooks, Cars), Fuel Cells (Space Shuttle), Photovoltaic Cells (Solar Panels), Electrolysis, Rust
- Topic: Identifying cathode & anode in electrolytic cells
- Replies: 4
- Views: 382
Re: Identifying cathode & anode in electrolytic cells
For electrolytic cells, the cathode is still the reaction getting reduced and the anode is the reaction being oxidized. You would still use the reduction cell potentials given but you will notice that when you calculate Ecell= E cathode -E anode you get a negative cell potential which further emphas...
- Sun Mar 01, 2020 10:00 pm
- Forum: Work, Gibbs Free Energy, Cell (Redox) Potentials
- Topic: n value
- Replies: 4
- Views: 396
Re: n value
The number of electrons exchanged should not change once the redox reaction is balanced.
- Sun Mar 01, 2020 9:52 pm
- Forum: Work, Gibbs Free Energy, Cell (Redox) Potentials
- Topic: when to use K or Q
- Replies: 18
- Views: 1117
Re: when to use K or Q
K is the equilibrium constant and therefore used at equilibrium. Q is used whenever the equation is not at equilibrium or when initial concentrations are given rather than equilibrium concentrations.
- Sun Mar 01, 2020 9:50 pm
- Forum: Work, Gibbs Free Energy, Cell (Redox) Potentials
- Topic: Reducing/oxidizing agent
- Replies: 8
- Views: 574
Re: Reducing/oxidizing agent
The reducing agent is the substance being oxidized since it is reducing the reduction reaction. The oxidizing agent is the substance undergoing reduction since its reduction or gain of electrons is causing the loss of the oxidation.
- Mon Feb 24, 2020 10:34 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: gibbs free
- Replies: 2
- Views: 243
Re: gibbs free
Table 4.J.1 explains how these are related in different conditions. The relationship with all three is factoring in a systems entropy, enthalpy and temperature values in order to give the energy of the system.
- Fri Feb 21, 2020 7:21 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Electrode
- Replies: 3
- Views: 305
Re: Electrode
If the reaction does not have a solid metal included, then you add platinum to either side of the cell diagram. However, like he showed in class today, the equation had a solid metal for only one reaction and therefore Copper solid was used to conduct the anode and the cathode having no conductor, p...
- Fri Feb 21, 2020 7:18 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Wmax
- Replies: 2
- Views: 176
Re: Wmax
Wmax is related to deltaG when Temperature and Pressure are constant since those are the conditions that need to be met for Gibbs free energy.
- Fri Feb 21, 2020 7:03 pm
- Forum: Balancing Redox Reactions
- Topic: Homework L3 part d
- Replies: 1
- Views: 131
Re: Homework L3 part d
Finding the half reactions shouldn't be any different, but when you have OH- you do neutralize it by adding an H to the opposite side. However, the homework problem just ends up using the reaction of O2 given in the Table 6.M.1
- Fri Feb 21, 2020 6:18 pm
- Forum: Balancing Redox Reactions
- Topic: Charge of oxygen
- Replies: 15
- Views: 764
Re: Charge of oxygen
The oxidation state of oxygen will always be -2.
- Mon Feb 17, 2020 11:57 pm
- Forum: Calculating Work of Expansion
- Topic: Reversible vs Irreversible
- Replies: 13
- Views: 886
Re: Reversible vs Irreversible
Reversible means the system is in equilibrium with the surroundings. Whatever happens to the surroundings will therefore affect the system. When it is irreversible it is not in equilibrium.
- Mon Feb 17, 2020 11:40 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: deltaG in relation to K
- Replies: 3
- Views: 187
Re: deltaG in relation to K
It has to do with the Vant Hoff equation relating the equilibrium constant with spontaneity. Therefore, when the K value changes, so does the spontaneity of the reaction process.
- Mon Feb 17, 2020 11:29 pm
- Forum: Administrative Questions and Class Announcements
- Topic: HW7
- Replies: 14
- Views: 832
Re: HW7
Vant Hoff equation problems and I imagine some redox reaction problems would be accepted. He only introduced electrochemistry recently so I think any remaining thermochemistry or thermodynamic problems are fine.
- Mon Feb 17, 2020 11:26 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Midterm Curve
- Replies: 45
- Views: 2277
Re: Midterm Curve
His syllabus says he does not curve, but I imagine there would only be a curve if a very low number of people pass on an exam.
- Mon Feb 17, 2020 11:21 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: ∆G = ∆Gº + RT lnQ
- Replies: 12
- Views: 2626
Re: ∆G = ∆Gº + RT lnQ
This equation relates equilibrium constant with Gibbs free energy. The 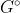 refers to finding the G value when the reactants and products are in their standard states.
refers to finding the G value when the reactants and products are in their standard states.
- Mon Feb 17, 2020 12:06 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Ph
- Replies: 8
- Views: 374
Re: Ph
pH is always calculated when the equation is at equilibrium. When pH is given it means that the H+ from the pH is the H+ value at equilibrium.
- Mon Feb 17, 2020 12:04 am
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: G vs G knot
- Replies: 15
- Views: 1742
Re: G vs G knot
G knot just means that it is only applicable when the elements in the equation are in their standard state since the knot denotes that it is the Gibbs free energy value at standard state.
- Mon Feb 17, 2020 12:02 am
- Forum: Concepts & Calculations Using Second Law of Thermodynamics
- Topic: Second Law Equation
- Replies: 1
- Views: 215
Second Law Equation
Is the equation 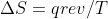 only used to find entropy of the surroundings or is it used to find the entropy of the system?
only used to find entropy of the surroundings or is it used to find the entropy of the system?
- Sun Feb 16, 2020 11:59 pm
- Forum: Calculating Standard Reaction Entropies (e.g. , Using Standard Molar Entropies)
- Topic: Hc vs Hf
- Replies: 5
- Views: 2718
Re: Hc vs Hf
Hf is used for standard enthalpy of formation for all equations, but Hc denotes enthalpy of combustion. This value would be helpful for a Hess's law calculation that is typical of combustion reactions.
- Sun Feb 16, 2020 11:55 pm
- Forum: Entropy Changes Due to Changes in Volume and Temperature
- Topic: Second law of thermodynamics
- Replies: 6
- Views: 511
Re: Second law of thermodynamics
The second law explains entropy in regards to a system. The total entropy of an isolated system can never decrease over time, and is constant if and only if all processes are reversible. This is used describe spontaneity in a system or if a reaction is favorable. The equations dealing with entropy h...
- Mon Feb 03, 2020 1:07 am
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: Cv vs Cp
- Replies: 3
- Views: 141
Re: Cv vs Cp
Cv and Cp are used only for gases to find their change of internal energy. There are calculation differences since gases are compressible and their Cp and Cv value will not be the same. Liquids and solids do not have these and we assume they have only one specific heat since they are incompressible.
- Mon Feb 03, 2020 12:59 am
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Standard Reaction Enthalpy
- Replies: 4
- Views: 123
Re: Standard Reaction Enthalpy
The standard enthalpy of formation is the change in enthalpy when one mole of a substance in the standard state (1 atm of pressure and 298.15 K) is formed from its pure elements under the same conditions. The standard enthalpy of reaction takes these values and calculates the heat given off or taken...
- Mon Feb 03, 2020 12:52 am
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: taking phase change into account
- Replies: 5
- Views: 177
Re: taking phase change into account
Phase change is taken into account when the matter is changing phase. For examples if a problem asks to find the energy from boiling water when you started at a certain temperature. You would calculate q=mc(Tf-Ti) and use the specific heat and then add the heat of vaporization energy which does not ...
- Mon Feb 03, 2020 12:47 am
- Forum: Thermodynamic Systems (Open, Closed, Isolated)
- Topic: Specific heat capacity
- Replies: 7
- Views: 449
Re: Specific heat capacity
They only differ in the units they use but they essentially calculate the same thing. Like example earlier in lecture, Lavelle used the molar heat capacity since the problem gave the substance in mol.
- Mon Feb 03, 2020 12:45 am
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Hess's Law
- Replies: 7
- Views: 540
Re: Hess's Law
Hess's law uses the step equations or reactions along with the energy transferred in each reaction to give you the overall delta H. It shows that enthalpy is a state function since it does not matter how many steps in between give the overall reaction since it will add up to the enthalpy of the over...
- Sun Jan 26, 2020 9:41 pm
- Forum: Phase Changes & Related Calculations
- Topic: Phase changes
- Replies: 8
- Views: 234
Re: Phase changes
In order for a phase change to happen enough heat or energy has to be applied to overcome the phase and then the bond. That is why when you use the second method you add the heat of fusion or vaporization to the bond enthalpy to get the enthalpy of the reaction.
- Sun Jan 26, 2020 9:39 pm
- Forum: Phase Changes & Related Calculations
- Topic: Enthalpy Calculations
- Replies: 5
- Views: 145
Re: Enthalpy Calculations
The only formula on the Constants and equations sheet is the third method equation. The rest will probably have to be memorized.
- Sun Jan 26, 2020 9:34 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Different Enthalpy Strategies
- Replies: 5
- Views: 187
Re: Different Enthalpy Strategies
They should all give you the net enthalpy or Hf. You should use them based on what information a question gives you.
- Sun Jan 26, 2020 9:28 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: method 3
- Replies: 2
- Views: 103
Re: method 3
The last method was just describing using the summation of the enthalpy of the products minus the summation of the reactants enthalpy will give you the overall enthalpy of the reaction. So it would be the moles of the reactants times their formation enthalpies (i.e. CH4+ 2O2 --> CO2+ 2H2O for summat...
- Sun Jan 26, 2020 9:21 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: 5% Rule
- Replies: 9
- Views: 359
Re: 5% Rule
The protonation being less than 5% guarantees that the assumption to neglect X because the change is so small so you should not have to use the quadratic.
- Sun Jan 19, 2020 10:19 pm
- Forum: Calculating pH or pOH for Strong & Weak Acids & Bases
- Topic: 6B. 3 Volume
- Replies: 2
- Views: 196
Re: 6B. 3 Volume
To find the desired pH you just use the desired concentration 0.025 and you get pH of 1.6. To get the actual solution pH you multiply the molarity by the 0.200 L volume in order to find the desired moles and divide that by 0.250 L to get the new molarity/H3O+ concentration. From there you put that i...
- Sun Jan 19, 2020 10:04 pm
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Buffers
- Replies: 3
- Views: 117
Re: Buffers
Buffers are solutions that when an acid or a base is added to the solution, it does not affect the pH. Buffers are usually made by adding a weak acid with its salt in water.
- Sun Jan 19, 2020 10:00 pm
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Weak acids & bases
- Replies: 7
- Views: 354
Re: Weak acids & bases
you divide the change number by the concentration of the acid. Then you multiply by 100 if you want the percent.
- Mon Jan 13, 2020 11:50 pm
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Temperature change
- Replies: 5
- Views: 119
Temperature change
Why does temperature change the K value, does it have to do with changing reaction rate?
- Sun Jan 12, 2020 9:14 pm
- Forum: Non-Equilibrium Conditions & The Reaction Quotient
- Topic: Response of Equilibria to Change
- Replies: 6
- Views: 160
Re: Response of Equilibria to Change
With any change the equilibrium will shift a certain way. A general rule is if there is more of reactant, the equilibrium will shift to the right (product) since it has more reactants to form product, and the same goes for an increase of product concentration: it will shift left to create the reacta...
- Sun Jan 12, 2020 9:06 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Concentration
- Replies: 5
- Views: 147
Re: Concentration
Since it is a constant, concentration of the product or reactant changing like for example adding more reactant or product will not change Kc, instead the system will shift accordingly to reach equilibrium again and that will happen when the system reaches the Kc ratio. The only thing that can affec...
- Sun Jan 12, 2020 9:04 pm
- Forum: Non-Equilibrium Conditions & The Reaction Quotient
- Topic: Pure Substances
- Replies: 3
- Views: 168
Re: Pure Substances
Solids and liquids are not added because their concentration does not affect the concentration of the reactants and therefore assumed to have a concentration of 1. Because of this, the 1 of their concentration is omitted from the equilibrium constant equation.
- Sun Jan 12, 2020 9:00 pm
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: volume change with inert gas
- Replies: 9
- Views: 258
Re: volume change with inert gas
The inert gas does not change the volume and therefore does not change the concentrations of the gases therefore it will not affect the equilibrium.
- Sun Jan 12, 2020 8:57 pm
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Le Chatelier's Principle
- Replies: 5
- Views: 244
Re: Le Chatelier's Principle
His principle states a change in pressure, temperature, or concentration of a reactant when applied to a system, the system will counteract the change and shift accordingly in order to return back to equilibrium.
- Sun Jan 12, 2020 8:55 pm
- Forum: Ideal Gases
- Topic: The Laws
- Replies: 7
- Views: 240
Re: The Laws
For now we should just know the ideal gas law equation and that gases are compressible.
- Sun Dec 08, 2019 9:28 pm
- Forum: Quantum Numbers and The H-Atom
- Topic: n, l ,ml, ms
- Replies: 13
- Views: 1513
Re: n, l ,ml, ms
When asked to write the ms of a certain configuration you just have to pick one of them since each refers to one electron spinning one way. Since there is no definite way to know you can pick one, +1/2 being up spin and -1/2 being down spin.
- Sun Dec 08, 2019 9:22 pm
- Forum: Naming
- Topic: [Fe(OH)(OH2)5]Cl2
- Replies: 3
- Views: 2594
Re: [Fe(OH)(OH2)5]Cl2
For finding charges in metals you have to know some fixed oxidation states and rules like H2O being a neutral molecule with a charge of 0 and OH always has a -1 charge.
- Sun Dec 08, 2019 9:18 pm
- Forum: Biological Examples
- Topic: vitamin b-12
- Replies: 3
- Views: 326
Re: vitamin b-12
It is one of the only metal ions that is able to bond with CH3 which is a biological molecule necessary in our system for many functions.
- Sun Dec 08, 2019 9:14 pm
- Forum: Conjugate Acids & Bases
- Topic: Acid/base reactions
- Replies: 3
- Views: 329
Re: Acid/base reactions
The lewis acid and base definition is used more for salts and compounds that do not have H ions or do not fit the definition of a bronsted acid or base. For a majority of acids and bases, the bronsted acid definition is used unless otherwise noted.
- Sun Dec 08, 2019 9:11 pm
- Forum: Polyprotic Acids & Bases
- Topic: H2S and HCl
- Replies: 2
- Views: 277
Re: H2S and HCl
Since Cl is more electronegative it will be able to ionize more easily since the bond is weaker. When it comes to acids and bases the ability to ionize is what matters since that is what will producing the pH or pOH.
- Sun Dec 08, 2019 9:09 pm
- Forum: Lewis Acids & Bases
- Topic: What does it mean when something is strong?
- Replies: 8
- Views: 618
Re: What does it mean when something is strong?
The strength of an acid is just based on their ability to ionize completely in water and therefore be able to produce H+ ions or OH- ions.
- Sun Dec 08, 2019 9:07 pm
- Forum: Quantum Numbers and The H-Atom
- Topic: The spin of a quantum number
- Replies: 6
- Views: 536
Re: The spin of a quantum number
There is no way to tell the spin of the electrons but in general you can use either +1/2 or -1/2. When they ask for quantum numbers you can use either or.
- Sun Dec 08, 2019 9:03 pm
- Forum: Wave Functions and s-, p-, d-, f- Orbitals
- Topic: atomic orbital probability
- Replies: 2
- Views: 393
Re: atomic orbital probability
This just refers to the Schrodinger equation which is what the orbitals are based off of. The equation basically explains why orbitals were used for interpretation.
- Sun Dec 08, 2019 9:00 pm
- Forum: General Science Questions
- Topic: Final : Question about Neutral or Ionized acid
- Replies: 3
- Views: 500
Re: Final : Question about Neutral or Ionized acid
Another way to check if it was ionized was to use the ph value and from that plug it in to the Ka equation. pH just refers to the H+ concentration and therefore you would know the value of H+ and A- and would be able to solve for the HA value which ended up being larger than the other concentrations.
- Sun Dec 08, 2019 8:58 pm
- Forum: Properties & Structures of Inorganic & Organic Bases
- Topic: Equilibrium sign
- Replies: 9
- Views: 906
Re: Equilibrium sign
Equilibrium sign is added only for weak acids and bases since they do not dissociate or ionize fully.
- Sun Dec 08, 2019 8:55 pm
- Forum: SI Units, Unit Conversions
- Topic: HELP WITH UNITS
- Replies: 9
- Views: 2347
Re: HELP WITH UNITS
It depends on the problem and what units the equation you are using uses. Sometimes it will give you a certain unit and the final question will ask you to convert it to a different unit.
- Sun Dec 01, 2019 4:52 pm
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: Oxidation Number
- Replies: 9
- Views: 778
Re: Oxidation Number
How would we know the oxidation number if the formula is just given? Certain ions have fixed oxidation states like oxygen is always (2-) and SO4 is always ((2-) and CN is always (-1) and so on. Molecules like H2O and gases like HOFBRINCl have an oxidation number of (0). https://d2jmvrsizmvf4x.cloud...
- Sun Dec 01, 2019 4:45 pm
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: Polydentates
- Replies: 1
- Views: 150
Re: Polydentates
Polydentates are just able to bind multiple molecules by having two or more lone pairs. A ligands ability to bind at multiple sites depends on its orbitals and number of electrons, therefore some ligands are only able to bind once and others are able to bind more. Chelating ligands bind cations tigh...
- Sun Dec 01, 2019 4:35 pm
- Forum: Naming
- Topic: Cis & Trans
- Replies: 3
- Views: 243
Re: Cis & Trans
It should be written out in order to specify the difference between the molecular structure. The molecule is the same therefore the only difference is the position of the molecules and the cis and trans prefixes specify which position you are referring to.
- Sun Dec 01, 2019 4:33 pm
- Forum: Properties & Structures of Inorganic & Organic Bases
- Topic: Metal Oxide and Water
- Replies: 1
- Views: 114
Re: Metal Oxide and Water
When a metal oxide reacts with water, a base is produced and OH- ions are also produced. Nonmetal oxides produce acids.
https://qph.fs.quoracdn.net/main-qimg-c ... 06f3907d49
https://qph.fs.quoracdn.net/main-qimg-c ... 06f3907d49
- Sun Dec 01, 2019 4:29 pm
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: Oxidation Number
- Replies: 9
- Views: 778
Re: Oxidation Number
The oxidation number should be written even when it is zero next to the metal because metals can have various oxidation states which is why it needs to be specified which oxidation state it is in.
- Sun Dec 01, 2019 4:20 pm
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: Coordination Number of a Specific Ligand
- Replies: 1
- Views: 126
Re: Coordination Number of a Specific Ligand
It's six because ethylene diamine I believe is considered two ligands therefore two of these would make it four and then plus the 2 Cl, it would give a coordination number of 6.
- Sun Nov 24, 2019 4:45 pm
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: Chelating ligands and cations
- Replies: 1
- Views: 136
Re: Chelating ligands and cations
Yes, it is due to the properties of the ring structure. The atoms are closer together therefore making the bond tighter with their electron density.
- Sun Nov 24, 2019 4:40 pm
- Forum: *Molecular Orbital Theory (Bond Order, Diamagnetism, Paramagnetism)
- Topic: pi and sigma bonds
- Replies: 4
- Views: 342
Re: pi and sigma bonds
Sigma bonds overlap and can rotate while pi bonds are directly next to each other and are not able to rotate.
- Sun Nov 24, 2019 4:39 pm
- Forum: Bronsted Acids & Bases
- Topic: Bronsted vs Lewis acids
- Replies: 5
- Views: 364
Re: Bronsted vs Lewis acids
Bronsteds says that acids are H atom donors and lewis acids are electron donors. Bronsted's definition limits acids only to compounds that have hydrogen atoms bonded to it while Lewis acids definition can be expanded to any ionic compound that has an atom donating an electron.
- Sun Nov 24, 2019 4:37 pm
- Forum: Naming
- Topic: coordination compounds
- Replies: 4
- Views: 249
Re: coordination compounds
Lavelle said to use the one that has the asterisk next to it but he also said in his email that either or is fine.
- Sun Nov 24, 2019 4:34 pm
- Forum: Properties & Structures of Inorganic & Organic Acids
- Topic: acids and hydrogen
- Replies: 1
- Views: 201
Re: acids and hydrogen
It depends on which definition of acid we are using since a Lewis acid is any compound that is an electron donor and Bronsted's definition is any compound that donates a hydrogen atom in water. For the most part, all acids should have a hydrogen bonded to them.
- Sun Nov 24, 2019 4:31 pm
- Forum: Naming
- Topic: Cyano vs cyanido
- Replies: 1
- Views: 67
Re: Cyano vs cyanido
It's just a difference in name. Cyano is the one you should use since it is the one Lavelle has asterisked on his website, but he said either or is fine. Cyanido is just the new IUPAC Name convention.
- Sat Nov 16, 2019 5:30 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Bond Angles
- Replies: 8
- Views: 645
Re: Bond Angles
The bond angles are just experimental observations, so some of the angles just have to be memorized. Lavelle said that we just have to know when to assume a bond angle will be less than another if it has more lone pairs, etc.
- Sat Nov 16, 2019 5:27 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: VSEPR and Polarity
- Replies: 4
- Views: 143
Re: VSEPR and Polarity
You look for which atoms are electronegative and see where they are placed in order to see if their dipoles cancel out or make the molecule polar.
- Sat Nov 16, 2019 5:25 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Melting Points
- Replies: 7
- Views: 484
Re: Melting Points
I don't think you have to memorize the melting points, but just know the strength of each molecular interaction and compare them.
- Sat Nov 16, 2019 5:20 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Different Types of Bonds
- Replies: 2
- Views: 210
Re: Different Types of Bonds
They are treated the same because they are all just an area of electron density no matter the bonds.
- Sat Nov 16, 2019 5:18 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Octahedral Arrangement
- Replies: 2
- Views: 203
Re: Octahedral Arrangement
The lewis structures are only showing the valence electrons which with the octet rule means 8 electrons in order for the atom to be stable. Expanding the octet with atoms that are able to just depends on the formal charge of the atoms and what would ultimately lead to a stable structure.
- Sun Nov 03, 2019 11:31 pm
- Forum: Bond Lengths & Energies
- Topic: Short bond lengths vs long bond lengths
- Replies: 6
- Views: 477
Re: Short bond lengths vs long bond lengths
Shorter bond lengths are stronger because there are more electrons to break and the electron density is higher.
- Sat Nov 02, 2019 12:00 am
- Forum: Bond Lengths & Energies
- Topic: Bond strength and electronegativity
- Replies: 2
- Views: 189
Re: Bond strength and electronegativity
Electronegativity describes the tendency of an atom to attract a shared pair of electrons towards itself. Bond strength increases as the difference in electronegativity between the atoms that are bonded increases.
- Fri Nov 01, 2019 11:48 pm
- Forum: Bohr Frequency Condition, H-Atom , Atomic Spectroscopy
- Topic: Rydberg Equation
- Replies: 2
- Views: 121
Re: Rydberg Equation
I believe for anything to do with electrons and electron emission I think he wants us to use this one En = - h R/n^2 . This is what is on the constants and equations sheet.
- Fri Nov 01, 2019 11:45 pm
- Forum: Bond Lengths & Energies
- Topic: Question 2.C.3.c
- Replies: 2
- Views: 110
Re: Question 2.C.3.c
It wouldn't bind with phosphorus because it would cause the formal charge to change to one that is energetically unfavorable. If the hydrogen was bonded to the phosphorus it would change to a formal charge of -1 rather than 0 which is what it has with 5 shared electron bonds. Additionally the hydrog...
- Fri Nov 01, 2019 11:23 pm
- Forum: Properties of Light
- Topic: when not to use the light equation?
- Replies: 4
- Views: 122
Re: when not to use the light equation?
You only use the light equation or any equations dealing with c which is speed of light when it has anything to do with EM radiation because the speed of light is a form of EM radiation which would have the same units as other energy from EM radiation. This is why DeBroglie is a separate equation be...
- Wed Oct 16, 2019 9:02 pm
- Forum: Wave Functions and s-, p-, d-, f- Orbitals
- Topic: What does the "x" indicate in (i.e.) the 2px state? [ENDORSED]
- Replies: 5
- Views: 405
Re: What does the "x" indicate in (i.e.) the 2px state? [ENDORSED]
The x indicates which plane the 2p orbital is in. The two orbitals can lie on the x, y, or z plane. https://qph.fs.quoracdn.net/main-qimg-5 ... 63712171dd
- Wed Oct 16, 2019 8:59 pm
- Forum: Properties of Electrons
- Topic: Question About Electron State
- Replies: 3
- Views: 116
Re: Question About Electron State
An electron in the 2px state just means its in the p orbital. The p orbital has three possible areas two electrons (2px,2py,2pz) so the x is just there to denote that its in the first p orbital.
- Wed Oct 16, 2019 8:55 pm
- Forum: Wave Functions and s-, p-, d-, f- Orbitals
- Topic: Magnetic Quantum number [ENDORSED]
- Replies: 4
- Views: 315
Magnetic Quantum number [ENDORSED]
Could someone explain how we are able to get 2,1,0,-1,-2 when we are finding the magnetic quantum numbers(ml) for the d orbitals. I know it goes l, l-1,..-l but how do we get -1 from this pattern? I am thinking 2,1,0(l-2), but would we get -1 from l-3 or something else? Could someone explain the pat...
- Mon Oct 14, 2019 7:09 pm
- Forum: Properties of Light
- Topic: 1A.15
- Replies: 4
- Views: 219
Re: 1A.15
I'm also having trouble with this problem. I found v using the equation v=c/λ and then used that in the equation ΔE= hv. However, from there I got a little lost. I used the equation En= -hR/ n^2 using n=1 to find Ei. I then plugged in ΔE and Ei into ΔE= Ef-EI to get Ef which I set equal to En= -hR/...
- Mon Oct 14, 2019 7:01 pm
- Forum: Wave Functions and s-, p-, d-, f- Orbitals
- Topic: Orbital equation
- Replies: 3
- Views: 102
Re: Orbital equation
The symbol he used here is rho so I don't think he was talking about orbitals here.
- Mon Oct 14, 2019 6:47 pm
- Forum: Wave Functions and s-, p-, d-, f- Orbitals
- Topic: Orbital equation
- Replies: 3
- Views: 102
Orbital equation
In the last few slides of lecture, Dr. Lavelle used this equation 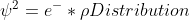
Does anyone know what the rho symbol stands for in this equation or what its value is?
Does anyone know what the rho symbol stands for in this equation or what its value is?
- Sat Oct 12, 2019 2:45 pm
- Forum: Properties of Light
- Topic: 1B3
- Replies: 3
- Views: 106
Re: 1B3
It should be photoelectric effect since it showed that light has discrete amount of energies called photons. It showed that no matter how the amplitude changed, the energy was still the same, but when the frequency was changed, the wavelength, it was able to displace electrons.
- Sat Oct 12, 2019 2:41 pm
- Forum: Properties of Light
- Topic: Equations
- Replies: 4
- Views: 163
Re: Equations
I believe the only one that doesn't apply to EM radiation is the De Broglie equation. The others deal with light therefore they are EM radiation equations.
- Sat Oct 12, 2019 2:40 pm
- Forum: DeBroglie Equation
- Topic: De Broglie's Equation
- Replies: 13
- Views: 569
Re: De Broglie's Equation
It works for any particle (object that has rest mass) with momentum. It is used for objects such as cars or baseballs as shown in the lecture which do not have detectable wavelengths. De Broglie claimed that all particles must have wave like properties.
- Sat Oct 12, 2019 2:33 pm
- Forum: Properties of Electrons
- Topic: Diffraction
- Replies: 4
- Views: 249
Re: Diffraction
The experiment that was shown in class and in the book where there were two cut outs where light was shined through and the light appeared where there was a wall in front. If light behaved like a particle it would shine through the cutout and appear right there, but it did not which meant that waves...
- Sat Oct 12, 2019 2:21 pm
- Forum: Properties of Light
- Topic: Help with 1A.11?
- Replies: 2
- Views: 132
Re: Help with 1A.11?
I believe the light emitted is just the difference between the frequency that displaced the atom and the threshold energy, so I don't know if its a higher frequency each time since sometimes it can be zero when it matches the threshold energy.
- Sat Oct 12, 2019 2:19 pm
- Forum: Properties of Electrons
- Topic: Properties of electron being Particle v Wave
- Replies: 3
- Views: 164
Re: Properties of electron being Particle v Wave
The photoelectric effect (i.e. shining light on a metal and finding the threshold energy of an electron) was used as strong evidence that light is a photon with certain energy/frequency. This proved that electromagnetic radiation including electrons behaved in a particle like matter rather than a wa...
- Sat Oct 12, 2019 2:07 pm
- Forum: Properties of Electrons
- Topic: wave properties of electrons
- Replies: 4
- Views: 162
Re: wave properties of electrons
If that were to happen then yes, the electron would exhibit particle like properties rather than wave like since 10^-15 is the cut off.
- Fri Oct 04, 2019 12:47 am
- Forum: Limiting Reactant Calculations
- Topic: Limiting Reactant
- Replies: 2
- Views: 175
Re: Limiting Reactant
I also had trouble with this one. First you would convert the 5 g to moles and then divide by the 150 ml to get the concentration. Since you are extracting from an already dissolved solution the new volume is the 20 ml. So, when you are doing M initial x V initial = M final x V final, you would use ...
- Wed Oct 02, 2019 9:22 pm
- Forum: SI Units, Unit Conversions
- Topic: Fundamentals E Question E.1
- Replies: 3
- Views: 189
Re: Fundamentals E Question E.1
If it doesn't specify, I would just go with what the problem is using. I would just go with the pm for measurement of length.
- Wed Oct 02, 2019 9:18 pm
- Forum: Balancing Chemical Reactions
- Topic: Balancing Chemical Reaction
- Replies: 6
- Views: 217
Re: Balancing Chemical Reaction
The problem wants you to write the chemical equation out yourself rather than provide it. This is a combustion reaction since butane is being burned, therefore for any combustion reaction the gas would react with O2 and produce CO2 and H2O. For this problem the equation would be 4C4H10 + O2 --> CO2 ...
- Wed Oct 02, 2019 3:52 pm
- Forum: Empirical & Molecular Formulas
- Topic: HW problem F9
- Replies: 8
- Views: 354
Re: HW problem F9
What do you mean atom ratio?

