A few moles of lithium hexafluorophosphate were sentenced to five years in prison.
They were charged with a salt in battery. :)
Search found 100 matches
- Sun Mar 15, 2020 9:36 pm
- Forum: Student Social/Study Group
- Topic: Post All Chemistry Jokes Here
- Replies: 9651
- Views: 3634168
- Sun Mar 15, 2020 9:30 pm
- Forum: Student Social/Study Group
- Topic: Post All Chemistry Jokes Here
- Replies: 9651
- Views: 3634168
Re: Post All Chemistry Jokes Here
What happens when oxygen atoms get haircuts?
They donate their lone hairs :)
They donate their lone hairs :)
- Sun Mar 15, 2020 9:23 pm
- Forum: Method of Initial Rates (To Determine n and k)
- Topic: Different methods
- Replies: 2
- Views: 386
Re: Different methods
Each approach varies in usefulness depending on the context of the problem, the complexity of the reaction, and what information is provided. The differential rate law approach expresses the reaction rate in terms of changes in the concentration of one or more reactants (Δ[R]) over a specific time i...
- Sun Mar 15, 2020 9:11 pm
- Forum: First Order Reactions
- Topic: Pseudo first order
- Replies: 2
- Views: 304
Re: Pseudo first order
A pseudo-first-order reaction is a reaction which is not a first-order reaction naturally, but is made first-order by increasing or decreasing the concentration of one or the other reactant. This article provides an in-depth explanation with examples: https://chem.libretexts.org/Bookshelves/Physical...
- Sun Mar 15, 2020 9:02 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Athena
- Replies: 34
- Views: 3186
Re: Athena
Thank you for everything you've done these past two quarters, Dr. Lavelle! I know this situation was very unexpected and difficult to manage, but I think you handled it wonderfully.
*high five* :)
*high five* :)
- Sun Mar 08, 2020 10:59 pm
- Forum: Second Order Reactions
- Topic: Second Order Reactions
- Replies: 5
- Views: 442
Re: Second Order Reactions
A second-order reaction (where order = 2) has a rate proportional to the concentration of the square of a single reactant or the product of the concentration of two reactants. The formula is: rate = k[A] 2 (or substitute B for A or k multiplied by the concentration of A times the concentration of B)...
- Sun Mar 08, 2020 10:53 pm
- Forum: Van't Hoff Equation
- Topic: deriving
- Replies: 3
- Views: 380
Re: deriving
If we covered the derivation in class, or it is mentioned specifically on the course outline (a few derivations are mentioned explicitly), they are "fair game", as Professor Lavelle likes to put it.
- Sun Mar 08, 2020 10:38 pm
- Forum: Kinetics vs. Thermodynamics Controlling a Reaction
- Topic: Versus Versus
- Replies: 2
- Views: 228
Re: Versus Versus
Thermodynamics is not about things moving and changing but instead about how stable they are in one state versus another, while kinetics is about how quickly or slowly species react. Thermodynamics-VERSUS-Kinetics.jpg I think you might need to take a look at the lesson outlines but here are some equ...
- Sun Mar 08, 2020 10:23 pm
- Forum: Biological Examples
- Topic: iN relation to ATP
- Replies: 3
- Views: 900
Re: iN relation to ATP
An article I read said that ATP could be analogously described and 'rechargeable batteries.' The batteries are used, giving up their potential energy until all of it has been converted into kinetic energy and heat(unusable energy). Recharged batteries can be used only after the input of additional e...
- Sun Mar 08, 2020 10:14 pm
- Forum: Interesting Applications: Rechargeable Batteries (Cell Phones, Notebooks, Cars), Fuel Cells (Space Shuttle), Photovoltaic Cells (Solar Panels), Electrolysis, Rust
- Topic: Electroplating
- Replies: 1
- Views: 311
Re: Electroplating
"Electroplating is a process that uses electric current to reduce dissolved metal ions by the use of electrolysis, to obtain the dissolved metal ions at the other electrode, mostly in the form of a uniform coating." This article explains the concept in more detail: https://byjus.com/physic...
- Sun Mar 01, 2020 6:39 pm
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: Why don't you have to convert pressure into concentration when doing lnQ?
- Replies: 3
- Views: 337
Re: Why don't you have to convert pressure into concentration when doing lnQ?
Unless it specifically asks for Qp or Qc, I don't think you need to convert anything. And typically you're going to be given partial pressures for gases and concentrations for aqueous solutions, which I believe was the case in the problem you provided as an example.
- Sun Mar 01, 2020 6:35 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: 6M.1
- Replies: 1
- Views: 213
Re: 6M.1
When solving this problem, you are given that the E° (cell potential) is equal to -0.689V Since we know that Cu/Cu 2+ is the anode, M 2+ /M is the cathode. Since E°= E° (cathode) - E° (anode) and the table gives us E° (anode) = +0.34V We just plug in the values: -0.689V = E° ...
- Sun Mar 01, 2020 6:26 pm
- Forum: Thermodynamic Systems (Open, Closed, Isolated)
- Topic: Heat Death
- Replies: 2
- Views: 436
Re: Heat Death
The idea of the universe's eventual heat death suggests that the universe will eventually evolve to a state of no thermodynamic free energy. Therefore, it would be unable to sustain processes that increase entropy. Once we reach this maximum entropy, no more work can be extracted from the universe. ...
- Sun Mar 01, 2020 4:33 pm
- Forum: Zero Order Reactions
- Topic: Order of Reactions
- Replies: 4
- Views: 445
Re: Order of Reactions
The order of reaction can be found experimentally by changing the concentration of reactants and observing the change in the rate of reaction. For example, if doubling the concentration of a reactant doubles the rate of reaction, the reaction is a first-order reaction for that reactant. rates.png ze...
- Sun Mar 01, 2020 4:25 pm
- Forum: Balancing Redox Reactions
- Topic: 6L.9 a)
- Replies: 1
- Views: 216
Re: 6L.9 a)
Usually, if you have something on both sides of the reaction (i.e. it is not being changed in any way and isn't really part of the reaction) you want to cancel it on both sides to simplify your final redox reaction.
- Sun Feb 23, 2020 10:42 pm
- Forum: Third Law of Thermodynamics (For a Unique Ground State (W=1): S -> 0 as T -> 0) and Calculations Using Boltzmann Equation for Entropy
- Topic: Boltzmann Equation
- Replies: 2
- Views: 274
Re: Boltzmann Equation
Boltzmann's equation is an equation relating the entropy S of an ideal gas to the quantity W, the number of real microstates corresponding to the gas' macrostate: S = kB ln W
where kB is the Boltzmann constant (also written as simply k) and equal to 1.38 × 10−23 J/K.
where kB is the Boltzmann constant (also written as simply k) and equal to 1.38 × 10−23 J/K.
- Sun Feb 23, 2020 10:37 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: 5J.11 b
- Replies: 2
- Views: 280
Re: 5J.11 b
This is because endothermic means a process or reaction that absorbs energy in the form of heat. To break a bond would require heat to be inputted as a reactant
- Sun Feb 23, 2020 10:31 pm
- Forum: Interesting Applications: Rechargeable Batteries (Cell Phones, Notebooks, Cars), Fuel Cells (Space Shuttle), Photovoltaic Cells (Solar Panels), Electrolysis, Rust
- Topic: Questions 6O.1 &6).3
- Replies: 1
- Views: 194
- Sun Feb 23, 2020 10:27 pm
- Forum: Work, Gibbs Free Energy, Cell (Redox) Potentials
- Topic: Vant Hoff Equation
- Replies: 9
- Views: 780
Re: Vant Hoff Equation
Most of the equations you'd need to know on an exam would be given on the constants/equation sheet, or could be derived from a different equation that is given. This article explains the Van't Hoff equation in a bit more detail: https://chem.libretexts.org/Bookshelves/Physical_and_Theoretical_Chemis...
- Sun Feb 23, 2020 9:52 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: salt bridge
- Replies: 9
- Views: 677
Re: salt bridge
The purpose of the salt bridge is to maintain charge balance as the electrons are moving from the anode to the cathode.
- Sun Feb 16, 2020 11:10 pm
- Forum: Calculating Standard Reaction Entropies (e.g. , Using Standard Molar Entropies)
- Topic: standard entropies vs. entropies
- Replies: 5
- Views: 577
Re: standard entropies vs. entropies
Standard entropy means the entropy of a certain substance under standard conditions. They would usually be given in a table format (like we saw on the midterm, we were given the standard molar entropy of different molecules) Here is a brief article explaining it: https://chem.libretexts.org/Bookshel...
- Sun Feb 16, 2020 11:05 pm
- Forum: Balancing Redox Reactions
- Topic: Why do we split equations?
- Replies: 12
- Views: 814
Re: Why do we split equations?
The short answer is that it makes it easier to balance the equation (keep in mind charge is important now as well as stoichiometric coefficients) Here is an article that goes more in-depth and provides some examples: https://opentextbc.ca/chemistry/chapter/17-1-balancing-oxidation-reduction-reaction...
- Sun Feb 16, 2020 10:58 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Heat Capacity for Calorimeters.
- Replies: 4
- Views: 483
Re: Heat Capacity for Calorimeters.
Heat capacity is a state function, but heat capacity is an intensive property. In contrast, specific heat capacity and molar heat capacity are extensive properties. Extensive means that it depends on the amount of material being affected, so mols or grams would be a factor in your calculations. heat...
- Sun Feb 16, 2020 10:51 pm
- Forum: Non-Equilibrium Conditions & The Reaction Quotient
- Topic: Writing a reaction for a buffer?
- Replies: 1
- Views: 258
Re: Writing a reaction for a buffer?
This video is an introduction, and some of the following videos in the series describe the actual calculations more in-depth.
https://www.khanacademy.org/science/chemistry/acid-base-equilibrium/buffer-solutions/v/buffer-system
https://www.khanacademy.org/science/chemistry/acid-base-equilibrium/buffer-solutions/v/buffer-system
- Sun Feb 16, 2020 10:48 pm
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Shift in Equilibrium
- Replies: 2
- Views: 137
Re: Shift in Equilibrium
This mainly depends on the substance that you add. For instance, an inert gas wouldn't affect the reaction at all. However, if you add something that reacts with either reactants or products, your system probably wouldn't remain at equilibrium because it would complicate the reaction and change the ...
- Sun Feb 09, 2020 8:19 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: State Function
- Replies: 7
- Views: 481
Re: State Function
Gibbs free energy is a state function since it's defined in terms of thermodynamic properties that are also state functions. Hess's law can be used for both changes in entropy and in Gibbs free energy.
- Sun Feb 09, 2020 8:16 pm
- Forum: Entropy Changes Due to Changes in Volume and Temperature
- Topic: Cp v.Cv
- Replies: 6
- Views: 347
Re: Cp v.Cv
C V is the molar specific heat at constant volume, and C P is the molar specific heat at constant pressure. So usually you will be told that a reaction is occurring at either constant pressure or constant volume, and you probably will be given the initial and final value if you are expected to use Δ...
- Sun Feb 09, 2020 8:10 pm
- Forum: Thermodynamic Definitions (isochoric/isometric, isothermal, isobaric)
- Topic: Entropy in reversible reactions
- Replies: 2
- Views: 92
Re: Entropy in reversible reactions
ΔS total =ΔS system +ΔS surrounding So if the ΔS total =0, then ΔS system = -ΔS surrounding They will not always be equal and opposite, but that is typically when this relationship will be important. Also, you could solve for ΔS system and ΔS surrounding and add if the question is asking for the ΔS ...
- Sun Feb 09, 2020 8:05 pm
- Forum: Concepts & Calculations Using Second Law of Thermodynamics
- Topic: Multistep Reactions
- Replies: 1
- Views: 97
Re: Multistep Reactions
Usually determining whether or not this is necessary will be a bit intuitive. In this particular problem, we were given a hint, but likely on an exam it will not be so obvious. Instead, take note of what information you are given and what you are being asked. For 12b, we were given the ΔH rxn = -275...
- Sun Feb 09, 2020 7:56 pm
- Forum: Thermodynamic Definitions (isochoric/isometric, isothermal, isobaric)
- Topic: Cs for monoatomic and diatomic
- Replies: 4
- Views: 220
Re: Cs for monoatomic and diatomic
Monatomic compounds are composed of single atoms, and diatomic compounds are composed of molecules containing two atoms. what-are-the-seven-diatomic-elements-606623-v3-5b562dab46e0fb0037fee8c7.png Difference-Between-Monatomic-and-Diatomic-Comparison-Summary.png I don't know why the C s values are 3/...
- Sun Feb 02, 2020 9:47 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Delta q and delta h
- Replies: 5
- Views: 312
Re: Delta q and delta h
Q is the amount of heat transferred to a system. ΔH is the change in enthalpy of a system in a reaction.
Q (heat) is not a state function. ΔH (change in enthalpy) is a state function.
Q (heat) is not a state function. ΔH (change in enthalpy) is a state function.
- Sun Feb 02, 2020 7:52 pm
- Forum: Third Law of Thermodynamics (For a Unique Ground State (W=1): S -> 0 as T -> 0) and Calculations Using Boltzmann Equation for Entropy
- Topic: Types of entropy
- Replies: 2
- Views: 100
Re: Types of entropy
Positional entropy is the number of molecular positions or arrangements that a system can have. This relates to the "dog bones" that we went over in last week's lecture
- Sun Feb 02, 2020 7:47 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: deltaU and deltaH
- Replies: 4
- Views: 197
Re: deltaU and deltaH
ΔH is the change in enthalpy and ΔU is the change in internal energy. Their relationship is explained by this equation: ΔH = ΔU + PΔV
- Sun Feb 02, 2020 7:44 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Constant pressure
- Replies: 2
- Views: 153
Re: Constant pressure
When a reaction is at constant pressure, the heat of reaction is equal to the enthalpy change of the system. Usually, you would be told there was a change in pressure, or it would be implied by the variables given.
- Sun Feb 02, 2020 7:34 pm
- Forum: Phase Changes & Related Calculations
- Topic: State functions
- Replies: 7
- Views: 451
Re: State functions
State functions are properties whose value does not depend on the path taken to reach that specific value. Some examples are internal energy, enthalpy, and entropy. However, things like heat and work aren't state functions, because they depend on the path taken from the initial to the final values. ...
- Sun Jan 26, 2020 3:36 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Calculating enthalpy change with phase changes
- Replies: 3
- Views: 118
Re: Calculating enthalpy change with phase changes
ΔHsub=ΔHfus+ΔHvap
If we know the enthalpies of certain phase changes, we can calculate an unknown value using what is given to us.
Some helpful diagrams:
If we know the enthalpies of certain phase changes, we can calculate an unknown value using what is given to us.
Some helpful diagrams:
- Sun Jan 26, 2020 3:24 pm
- Forum: Phase Changes & Related Calculations
- Topic: DeltaHsub
- Replies: 2
- Views: 165
Re: DeltaHsub
The reason that the ΔH sublimation is equal to the ΔH fusion + ΔH vaporization is that sublimation is the transition from the solid phase to the gas phase without passing through an intermediate liquid phase. So, from this, we can infer that adding the ΔH of the transition from solid to liquid and t...
- Sun Jan 26, 2020 3:17 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: bond v. standard
- Replies: 5
- Views: 319
Re: bond v. standard
Bond enthalpy is the energy needed to break a bond like C-H. Enthalpy of formation is the energy needed to form a substance from its pure components.
- Sun Jan 26, 2020 3:14 pm
- Forum: Phase Changes & Related Calculations
- Topic: State property
- Replies: 6
- Views: 301
Re: State property
A state property is a quantity that is independent of how the substance arrived at that state. Examples of state properties are altitude, pressure, volume, temperature and internal energy. A good way to imagine this is Dr. Lavelle's mountain example of the mountain, where one group takes a longer pa...
- Sun Jan 26, 2020 12:54 pm
- Forum: Phase Changes & Related Calculations
- Topic: Superheating and Supercooling
- Replies: 2
- Views: 152
Re: Superheating and Supercooling
Superheating and supercooling are very rare, as superheating is when something is heated past its boiling point without boiling/vaporizing. Similarly, supercooling is when an object is cooled below its freezing point without becoming a solid.
- Sun Jan 19, 2020 8:40 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: 6B.11
- Replies: 2
- Views: 128
Re: 6B.11
The sodium oxide (Na2O) is acting as a base, so sodium hydroxide (NaOH) is formed. It wouldn't form hydroxide and hydronium (H3+ and OH-)
- Sun Jan 19, 2020 8:31 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: 6A . 3
- Replies: 1
- Views: 154
Re: 6A . 3
Here are some simple examples. Basically you have a acid or base in your reactants (remember, water can be both and is usually the second reactant) and the conjugate base is created by the acid donating a proton, and the conjugate acid is created by the base gains a proton. conjugate.png conjugate 2...
- Sun Jan 19, 2020 8:23 pm
- Forum: Ideal Gases
- Topic: Using PV=nRT
- Replies: 7
- Views: 220
Re: Using PV=nRT
Everything that we have covered so far is fair game, so I would not be surprised if at least one question required this formula. However, if I remember correctly, it is on the equation sheet.
- Sun Jan 19, 2020 2:17 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: 6B. 11a)
- Replies: 2
- Views: 88
Re: 6B. 11a)
Since we figure out in part one that 10 -.75 =.018 mol⋅L -1 (.75 is the pOH and 10 -pOH undoes the log) we have to keep in mind that 5.00 mL of solution is added to the volumetric flask and diluted to 500.0 mL, so to find the molarity of the diluted solution in the second flask we have to take into ...
- Sun Jan 19, 2020 1:28 pm
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: salt pH
- Replies: 2
- Views: 202
Re: salt pH
This website explains everything step-by-step: https://courses.lumenlearning.com/cheminter/chapter/calculating-ph-of-salt-solutions/
- Sun Jan 12, 2020 8:30 pm
- Forum: Ideal Gases
- Topic: The Ideal Gas Law
- Replies: 3
- Views: 131
Re: The Ideal Gas Law
With the Ideal gas law, we assume things that we know are not true in a natural setting. For example, we'd ignore the volume that the ideal gas molecules would take up, and ignore intermolecular forces. However, outside of this idealized version of the situation, volume will be taken up by atoms and...
- Sun Jan 12, 2020 8:23 pm
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Practice Problems
- Replies: 4
- Views: 474
Re: Practice Problems
There is actually a Khan Academy section that has some video lessons and practice problems related to Le Chatlier's Principle
https://www.khanacademy.org/science/chemistry/chemical-equilibrium/factors-that-affect-chemical-equilibrium/v/le-chatelier-s-principle
https://www.khanacademy.org/science/chemistry/chemical-equilibrium/factors-that-affect-chemical-equilibrium/v/le-chatelier-s-principle
- Sun Jan 12, 2020 8:19 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Comparing K
- Replies: 9
- Views: 271
Re: Comparing K
Since K= [P]/[R], having a value of K>1 means that there is more product than reactant. Similarly, K<1 means there is more reactant than product. If K is small (K<10 -3 ) there are more reactants at equilibrium, and we would say that the equilibrium is "sitting to the left" If K is large (...
- Sun Jan 12, 2020 8:09 pm
- Forum: Ideal Gases
- Topic: Ideal gases equation
- Replies: 3
- Views: 257
Re: Ideal gases equation
n is the number of moles.
- Sun Jan 12, 2020 8:08 pm
- Forum: Ideal Gases
- Topic: Vapor Pressure
- Replies: 4
- Views: 197
Re: Vapor Pressure
In a sealed container, when a gas and its liquid form are at equilibrium, vapor pressure is the pressure of the gas above the liquid. Here is a video that explains it a bit more: https://www.khanacademy.org/science/chemistry/gases-and-kinetic-molecular-theory/ideal-gas-laws/v/vapor-pressure-example ...
- Sat Dec 07, 2019 11:41 pm
- Forum: Interionic and Intermolecular Forces (Ion-Ion, Ion-Dipole, Dipole-Dipole, Dipole-Induced Dipole, Dispersion/Induced Dipole-Induced Dipole/London Forces, Hydrogen Bonding)
- Topic: strengths
- Replies: 4
- Views: 372
Re: strengths
Here is a helpful diagram:
- Sat Dec 07, 2019 11:37 pm
- Forum: Coordinate Covalent Bonds
- Topic: Cisplatin
- Replies: 3
- Views: 314
Re: Cisplatin
Another thing to note is that cisplatin is effective and transplatin is not because a cisplatin molecule has chlorines on the same side, which can bond with two adjacent guanines. This creates a stronger hold, and prevents replication. Transplatin couldn't because the chlorines are on opposite sides...
- Sat Dec 07, 2019 11:27 pm
- Forum: Lewis Acids & Bases
- Topic: how to identify lewis acids
- Replies: 3
- Views: 356
Re: how to identify lewis acids
Hopefully this example will help: The boron atom in boron trifluoride, BF3, only has six valence electrons. Since it does not have a complete octet, BF3 is a Lewis acid and reacts with many Lewis bases. In this case, a fluoride ion acts as the Lewis base and donates one of its lone pairs. lewis acid...
- Sat Dec 07, 2019 11:23 pm
- Forum: Properties & Structures of Inorganic & Organic Acids
- Topic: different acids
- Replies: 1
- Views: 193
Re: different acids
Anything given in lecture or written in the learning outcomes is "fair game", as Dr. Lavelle puts it. It would be beneficial to know the common formulas.
- Sat Dec 07, 2019 11:21 pm
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: dentates
- Replies: 2
- Views: 237
Re: dentates
The polydentate ligands form coordinate bonds with the central metal atom at multiple sites (implied by the number of the prefix) and monodentate would only form one coordinate bond
- Sat Dec 07, 2019 10:20 pm
- Forum: Lewis Acids & Bases
- Topic: Lewis vs Bronsted
- Replies: 7
- Views: 638
Re: Lewis vs Bronsted
A lewis acid accepts an electron pair, while a lewis base donates an electron pair.
Bronsted/Lowry acids donate a proton and the bronsted/lowry base accepts a proton.
Bronsted/Lowry acids donate a proton and the bronsted/lowry base accepts a proton.
- Sat Dec 07, 2019 10:18 pm
- Forum: Lewis Acids & Bases
- Topic: Strong/Weak Acids and Bases
- Replies: 4
- Views: 503
- Sun Dec 01, 2019 8:13 pm
- Forum: Properties & Structures of Inorganic & Organic Bases
- Topic: Inorganic and organic
- Replies: 3
- Views: 239
Re: Inorganic and organic
Organic compounds always include carbon, while typically inorganic compounds do not. Also, most organic compounds contain C-H bonds. Keep in mind that simply containing carbon is not a sufficient explanation for a compound being organic. Molecules associated with living organisms are organic. These ...
- Sun Dec 01, 2019 8:06 pm
- Forum: *Crystal Field Theory
- Topic: Crystal Field Theory
- Replies: 2
- Views: 804
Re: Crystal Field Theory
Crystal Field Theory is a model for the bonding interactions between transition metals and ligands. However, I'm fairly sure this topic is not in the Learning Outcomes Outline, so you shouldn't expect to encounter it on the final. I would definitely double-check on Lavelle's website if you're not su...
- Sun Dec 01, 2019 8:01 pm
- Forum: Amphoteric Compounds
- Topic: Amphoteric Compound
- Replies: 5
- Views: 615
Re: Amphoteric Compound
Amphoteric compounds are molecules or ions that could react as either an acid or a base. Many metals like copper, zinc, tin, lead, aluminium, and beryllium form these amphoteric oxides or hydroxides.
A specific example of one would be Al2O3 (Aluminum Oxide), which is an amphoteric oxide.
A specific example of one would be Al2O3 (Aluminum Oxide), which is an amphoteric oxide.
- Sun Dec 01, 2019 7:58 pm
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: coordination sphere
- Replies: 1
- Views: 164
Re: coordination sphere
The coordination sphere is basically just the central atom/ion and the surrounding molecules or anions and ligands.
Usually if it's written out it will be within brackets
Usually if it's written out it will be within brackets
- Sun Dec 01, 2019 5:49 pm
- Forum: Lewis Acids & Bases
- Topic: oxoacids
- Replies: 1
- Views: 128
Re: oxoacids
Oxoacids are acids that contain oxygen, another element, and have at least one hydrogen bonded to the oxygen. The acid strength increases when the central atom is unchanged and the amount of oxygens surrounding it increases. Also, with an unchanged amount of oxygen, the strength of the acid increase...
- Mon Nov 18, 2019 7:04 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: 4.5
- Replies: 4
- Views: 954
Re: 4.5
Camille 4I wrote:Do lone pairs have more repulsion than double bonds?
Yes, the strength of repulsion goes
lone pair-lone pair > lone pair-bonding pair > bonding pair-bonding pair
- Sun Nov 17, 2019 6:29 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: VSEPR
- Replies: 3
- Views: 227
Re: VSEPR
The VSEPR model is used to determine shape by taking into account the regions of electron density, and which regions will have stronger repulsions. This is why knowing the lone pairs is important. You can estimate the bond angles, but VSEPR is not 100% accurate so experimental observation is necessa...
- Sun Nov 17, 2019 6:25 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Shapes
- Replies: 7
- Views: 457
Re: Shapes
The groups of Electron Geometry are: Linear (2 regions e- density) linear.PNG Trigonal Planar (3 regions e- density) trigonal planar.PNG Tetrahedral (4 regions e- density) tetrahedral.PNG Trigonal-Bipyramidal (5 regions e- density) trigonal-bypyramidal.PNG and Octahedral (6 regions e- density) I co...
- Sun Nov 17, 2019 6:24 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Shapes
- Replies: 7
- Views: 457
Re: Shapes
The groups of Electron Geometry are: Linear (2 regions e- density) linear.PNG Trigonal Planar (3 regions e- density) trigonal planar.PNG Tetrahedral (4 regions e- density) tetrahedral.PNG Trigonal-Bipyramidal (5 regions e- density) trigonal-bypyramidal.PNG and Octahedral (6 regions e- density)
- Sun Nov 17, 2019 6:13 pm
- Forum: Hybridization
- Topic: Orbitals
- Replies: 1
- Views: 139
Re: Orbitals
Not all orbitals in a molecule become hybridized during bonding, however, when you are trying to figure out the hybridized orbital, you must take into account every region of electron density surrounding the atom. VSEPR is actually predicting these hybridized shapes, and then the hybridized orbitals...
- Sun Nov 17, 2019 6:05 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: 2E.19A
- Replies: 2
- Views: 158
Re: 2E.19A
In terms of molecular shape, there would not be a difference because you'd have the same number of regions of electron density. Dr. Lavelle highlighted this in lecture when he was explaining why his non-ideal Lewis Structures were okay when doing VSEPR problems. However, if the question ever mention...
- Sun Nov 17, 2019 5:55 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: single, double, and triple bonds
- Replies: 6
- Views: 465
Re: single, double, and triple bonds
The double and triple bonds are also treated as one region of electron density because, although they have more electrons that a lone pair or single bond, the electrons lie in the same region (between the two bonded atoms) and they are oriented in the same direction. This means that it is technicall...
- Sun Nov 10, 2019 9:40 pm
- Forum: Sigma & Pi Bonds
- Topic: Sigma vs pi bond
- Replies: 3
- Views: 191
Re: Sigma vs pi bond
Only p orbitals can form π bonds and σ bonds are formed by the overlap of atomic orbitals over the internuclear axis. π bonds are oriented perpendicularly to the internuclear axis. S and P orbitals can form different hybridizations, s + p → sp s + p + p → sp² s + p + p + p → sp³ Overlapping hybridiz...
- Sun Nov 10, 2019 9:16 pm
- Forum: Octet Exceptions
- Topic: incomplete octet
- Replies: 6
- Views: 495
Re: incomplete octet
Usually, the elements that do this are B, Al, Li, and H. B and Al will usually form compounds where they only have six electrons instead of a complete octet.
- Mon Nov 04, 2019 5:41 pm
- Forum: Ionic & Covalent Bonds
- Topic: Names and chemical formulas
- Replies: 5
- Views: 448
Re: Names and chemical formulas
It is possible that we will encounter common molecules and the formula will not be given.
Here is a helpful Quizlet to memorize them: https://quizlet.com/_3rd3b
Here is a helpful Quizlet to memorize them: https://quizlet.com/_3rd3b
- Mon Nov 04, 2019 5:31 pm
- Forum: *Shrodinger Equation
- Topic: Shrodinger equation
- Replies: 7
- Views: 521
Re: Shrodinger equation
I believe the reason we covered the Schrödinger equation so briefly is that Dr. Lavelle doesn't really like this equation (I'm pretty sure one of the UA's said that) so we most likely won't encounter any problems where it will be necessary. Also, any equation we need will be given on the equation co...
- Mon Nov 04, 2019 5:19 pm
- Forum: Lewis Structures
- Topic: Homework 2C3
- Replies: 1
- Views: 118
Re: Homework 2C3
When I asked an undergraduate assistant about this, they said that typically the chemical formula would be given, and on the rare occasion you get a question where the name is given and the formula is not, it will either be a very common compound or it will be obvious from the name. For example, pho...
- Sun Nov 03, 2019 10:20 pm
- Forum: Bond Lengths & Energies
- Topic: Dino Nugget Mini review
- Replies: 2
- Views: 226
Re: Dino Nugget Mini review
They posted the answers on the original Dino Nugget thread but here
- Sun Nov 03, 2019 10:11 pm
- Forum: Ionic & Covalent Bonds
- Topic: Covalent Character
- Replies: 3
- Views: 246
Re: Covalent Character
Covalent character is referring to when an ionic bond takes on some covalent characteristics because electron distortion pulls electrons into the bonding area, so they are partially shared. I don't think we've discussed Fajan's rule yet but this explains the reasoning behind the phenomenon more (it ...
- Sun Nov 03, 2019 10:03 pm
- Forum: Polarisability of Anions, The Polarizing Power of Cations
- Topic: Polarizability and Covalent Character
- Replies: 5
- Views: 386
Re: Polarizability and Covalent Character
Polarizability is referring to how easily displaced an atom's electrons are by neighboring atoms. In an ionic bond, ions which cause large distortions are referred to as having 'high polarizing power'. This distorts the electron density of an ion, so the electrons are being pulled into the bonding r...
- Sun Nov 03, 2019 9:49 pm
- Forum: Dipole Moments
- Topic: Dipole
- Replies: 1
- Views: 126
Re: Dipole
It's basically when the atoms in a molecule share electrons unequally.
μ = Q d
measured in debyes.
This video explains very nicely: https://www.youtube.com/watch?v=TyFd94TbdXU
μ = Q d
measured in debyes.
This video explains very nicely: https://www.youtube.com/watch?v=TyFd94TbdXU
- Sun Nov 03, 2019 9:44 pm
- Forum: Lewis Structures
- Topic: "Most favorable" Diagram
- Replies: 4
- Views: 412
Re: "Most favorable" Diagram
When referring to "most favorable", you want as many formal charges to = 0 as possible. Also, for instance, if you're drawing an ion with a net charge that is, for example, -1, your formal charges should add up to the net charge of the ion. Formal charges of 0 would indicate a more stable,...
- Sun Oct 27, 2019 9:19 pm
- Forum: Lewis Structures
- Topic: 2 Odd Configurations
- Replies: 4
- Views: 160
Re: 2 Odd Configurations
It's best to memorize exceptions to the rule because during the test you can use the rule to figure out every other answer. If we covered it in lecture, there is a good chance we will encounter it on a future test.
- Sun Oct 27, 2019 9:14 pm
- Forum: Ionic & Covalent Bonds
- Topic: 2A 15
- Replies: 2
- Views: 161
Re: 2A 15
Yes, you're on the right track
- Sun Oct 27, 2019 9:02 pm
- Forum: Resonance Structures
- Topic: Resonance Structures
- Replies: 4
- Views: 179
Re: Resonance Structures
I don't believe there is a particular formula for determining the number of resonance structures, so you would count the number of possible resonance structures by drawing every valid lewis structure until there aren't any possible alternative configurations.
- Sun Oct 27, 2019 8:38 pm
- Forum: Bond Lengths & Energies
- Topic: bond lengths
- Replies: 1
- Views: 163
Re: bond lengths
You can estimate bond lengths by adding the covalent radii of the two atoms, but this is not entirely accurate and experimental observation is always the better option.
- Sun Oct 27, 2019 8:25 pm
- Forum: Lewis Structures
- Topic: Drawing Lewis Structures
- Replies: 5
- Views: 185
Re: Drawing Lewis Structures
This video explains the idea very simply: https://www.youtube.com/watch?v=cIuXl7o6mAw
- Sun Oct 20, 2019 11:59 pm
- Forum: Quantum Numbers and The H-Atom
- Topic: orientation of orbitals
- Replies: 4
- Views: 732
Re: orientation of orbitals
Does this image help?
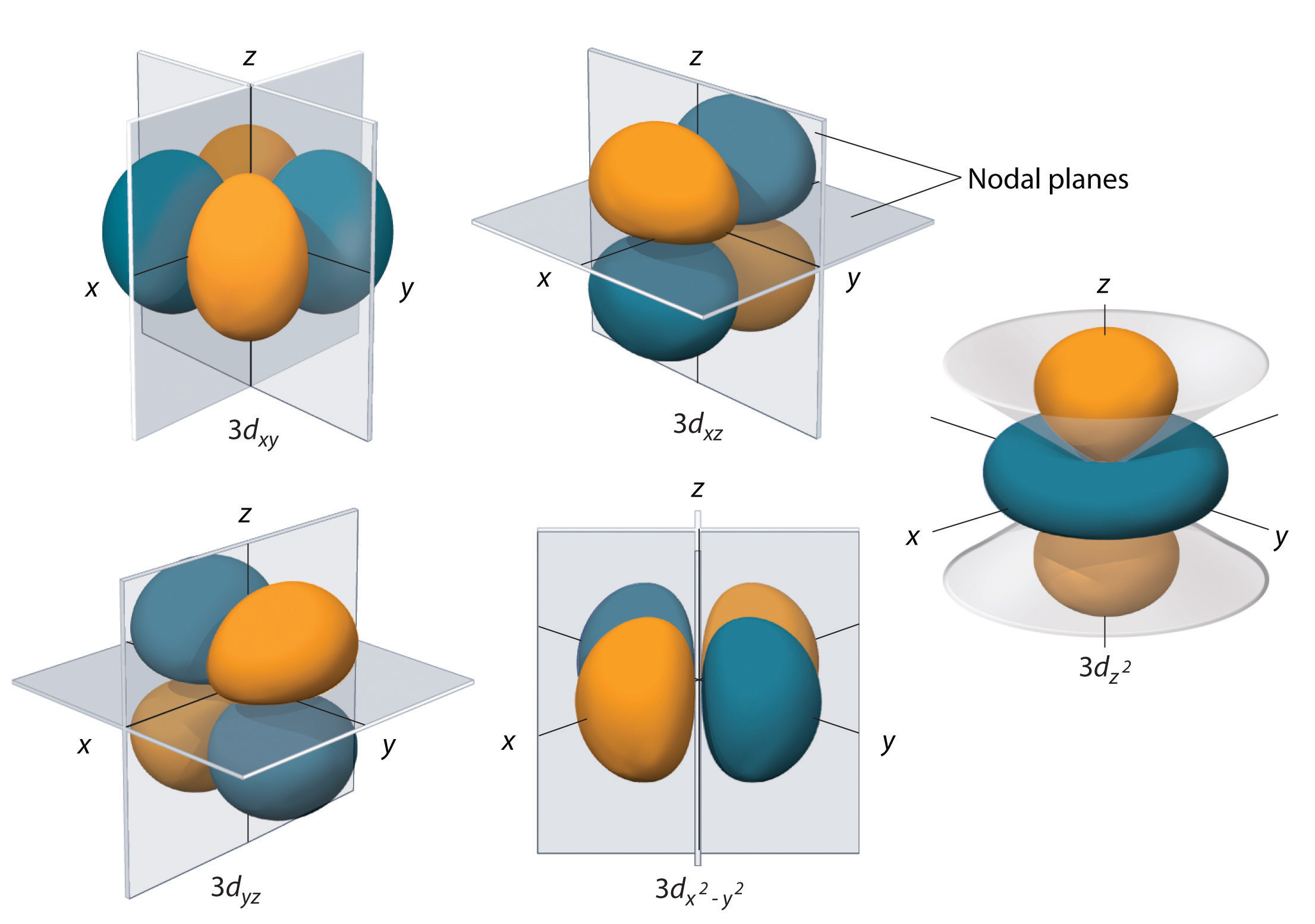

- Sun Oct 20, 2019 11:56 pm
- Forum: Electron Configurations for Multi-Electron Atoms
- Topic: Nodal Planes
- Replies: 2
- Views: 149
Re: Nodal Planes
A nodal plane is a plane where the probability of finding an electron is zero. You can find the coordinates of these planes by solving the Schrödinger wave equation
- Sun Oct 20, 2019 11:50 pm
- Forum: Quantum Numbers and The H-Atom
- Topic: Quantum Numbers and Energy Levels
- Replies: 2
- Views: 134
Re: Quantum Numbers and Energy Levels
The value of the energy level is proportional to 1/n^2 which gives a series 1, 1/4, 1/9, 1/16, and so on
- Sun Oct 20, 2019 11:41 pm
- Forum: Trends in The Periodic Table
- Topic: memorization
- Replies: 3
- Views: 294
Re: memorization
Here is a quick video that explains it:
https://youtu.be/XK-WTYncldA
https://youtu.be/XK-WTYncldA
- Sun Oct 20, 2019 11:27 pm
- Forum: Properties of Light
- Topic: 1B. 9
- Replies: 2
- Views: 137
Re: 1B. 9
You find the Energy per photon with E = hc/λ, and divide the 64J by the energy per photon to find out the number of photons, then you can multiply by Planck's constant to find the mols of photons
- Sun Oct 13, 2019 8:35 pm
- Forum: Properties of Electrons
- Topic: Mass of a neutron (1B.23)
- Replies: 2
- Views: 149
Re: Mass of a neutron (1B.23)
The mass of a neutron is 1.6749286*10^-27 kg
- Sun Oct 13, 2019 8:17 pm
- Forum: Properties of Light
- Topic: Helpful videos
- Replies: 1
- Views: 91
Re: Helpful videos
Crash Course Part 1 [youtube]https://www.youtube.com/watch?v=7kb1VT0J3DE[/youtube]
Crash Course Part 2 [youtube]https://www.youtube.com/watch?v=qO_W70VegbQ[/youtube]
Introduction (pretty boring but covers basic concepts) [youtube]https://www.youtube.com/watch?v=HC81oYe43DI[/youtube]
Crash Course Part 2 [youtube]https://www.youtube.com/watch?v=qO_W70VegbQ[/youtube]
Introduction (pretty boring but covers basic concepts) [youtube]https://www.youtube.com/watch?v=HC81oYe43DI[/youtube]
- Sun Oct 13, 2019 8:12 pm
- Forum: Properties of Electrons
- Topic: Photoelectric Effect
- Replies: 3
- Views: 174
Re: Photoelectric Effect
Electrons aren't emitted unless the energy of the photon is greater than or equal to the energy to remove an electron, even for high-intensity light. This outcome was unexpected, because the light was no longer behaving like a wave, otherwise simply raising the intensity would have been sufficient t...
- Sun Oct 13, 2019 8:05 pm
- Forum: Properties of Light
- Topic: Intensity & Amplitude
- Replies: 7
- Views: 467
Re: Intensity & Amplitude
Intensity is proportional to the square of amplitude. So, as a general rule, as the amplitude of a wave increases, so will the intensity.
- Sun Oct 13, 2019 7:58 pm
- Forum: SI Units, Unit Conversions
- Topic: knowing how many sig figs to use
- Replies: 17
- Views: 810
Re: knowing how many sig figs to use
To keep your work as accurate as possible, try not to round until the final answer. On the basis that you do round, keeping a certain number of significant figures should be enough to result in the final answer. For example, if the answer needs to have two significant figures, keeping four signific...
- Sun Oct 06, 2019 9:18 pm
- Forum: Administrative Questions and Class Announcements
- Topic: KAREN SUN 5-7PM WORKSHOP - DOWNLAOD WORKSHEETS HERE
- Replies: 53
- Views: 5978
Re: KAREN SUN 5-7PM WORKSHOP - DOWNLAOD WORKSHEETS HERE
Do we have to sign up for your workshops or are students welcome to just show up?
- Sun Oct 06, 2019 9:13 pm
- Forum: Student Social/Study Group
- Topic: Final Jitters
- Replies: 457
- Views: 371802
Re: Final Jitters
Before the test, you definitely want to make sure you are in the right head space. If you know you have an exam coming up, make an effort to get sleep and eat a good breakfast. Give yourself adequate time to study in advance so you do not feel like you are cramming the night before. Some light revie...
- Sun Oct 06, 2019 9:07 pm
- Forum: Significant Figures
- Topic: Reviewing Sig Figs
- Replies: 3
- Views: 161
Reviewing Sig Figs
I'm a bit rusty on sig figs since I haven't taken chem in a few years, does anybody have any specific resources for practicing that they found helpful? I learn better from practicing than memorizing rules and definitions.
- Sun Oct 06, 2019 9:03 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Advice from a Medical Student [ENDORSED]
- Replies: 297
- Views: 418473
Re: Advice from a Medical Student [ENDORSED]
Do you have any advice for professors who are not as accommodating as Lavelle? Did you ever struggle in a specific class because of a lack of resources/support?
- Sun Oct 06, 2019 8:58 pm
- Forum: General Science Questions
- Topic: Rusty on High School Chem [ENDORSED]
- Replies: 347
- Views: 439364
Re: Rusty on High School Chem [ENDORSED]
Its been a while for me too! I know this is basic, but knowing the common ions and compounds is so important to setting up problems and writing equations, but our course reader doesn't include much about them. I have this review list that will totally help!Common Ions.docx Thank you, I have been st...
- Sun Oct 06, 2019 8:50 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Advice from a Medical Student - Part II [ENDORSED]
- Replies: 298
- Views: 270757
Re: Advice from a Medical Student - Part II [ENDORSED]
Thank you for sharing your experience. I am planning to go to medical school, and it is comforting to hear from people who have been in my situation and are doing well.

