Search found 102 matches
- Thu Mar 12, 2020 6:26 pm
- Forum: Ideal Gases
- Topic: reversing reactions
- Replies: 83
- Views: 5233
Re: reversing reactions
The k for a reversed reaction is 1/k
- Thu Mar 12, 2020 6:23 pm
- Forum: Kinetics vs. Thermodynamics Controlling a Reaction
- Topic: What was your favorite chem topic?
- Replies: 137
- Views: 10935
What was your favorite chem topic?
After taking Dr. Lavelle's 14A and 14B class, what was your favorite chem topic so far?
- Thu Mar 12, 2020 6:20 pm
- Forum: Arrhenius Equation, Activation Energies, Catalysts
- Topic: Collision Theory, Mean Relative Speed, Transition State Theory
- Replies: 1
- Views: 160
Collision Theory, Mean Relative Speed, Transition State Theory
Do we need to know about the collision theory and the equations associated with it that are in the textbook?
- Thu Mar 12, 2020 11:01 am
- Forum: Second Order Reactions
- Topic: Graphs
- Replies: 9
- Views: 797
Graphs
Can someone explain and summarize what the graphs for each order reaction looks like and what they mean?
- Wed Mar 11, 2020 9:23 pm
- Forum: Second Order Reactions
- Topic: 7B.13
- Replies: 2
- Views: 345
7B.13
The half-life of A in a second-order reaction is 50.5 s when [A]0 = 0.84 mol/L. Calculate the time needed for the concentration of A to decrease to (a) one-sixteenth (b) one-fourth; (c) one-fifth of its original value.
Can someone please explain this problem
Can someone please explain this problem
- Tue Mar 03, 2020 11:12 pm
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: 6N.15
- Replies: 1
- Views: 219
6N.15
Calculate the potential of a cell constructed with two nickel electrodes. The electrolyte in one compartment is 1.0 m Ni(NO3)2(aq). In the other compartment, NaOH has been added to a Ni(NO3)2 solution until the pH = 11.0 at 298 K. See Table 6I.1. How do you determine which is the anode and which is ...
- Tue Mar 03, 2020 10:25 pm
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: 6N.9
- Replies: 1
- Views: 236
6N.9
A tin electrode in 0.015 m Sn(NO3)2(aq) is connected to a hydrogen electrode in which the pressure of H2 is 1.0 bar. If the cell potential is 0.061 V at 25 C, what is the pH of the electrolyte at the hydrogen electrode?
Where should you start with this problem?
Where should you start with this problem?
- Tue Mar 03, 2020 10:19 am
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: 6M.7
- Replies: 5
- Views: 398
6M.7
This question asks us to order metals by increasing strength as a reducing agent but how do we determine this? I know that the more negative the standard reduction potential, the stronger it would be as a reducing agent but when I looked up the reduction potentials in the back of the book, there wer...
- Mon Mar 02, 2020 7:26 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: 6M.1
- Replies: 5
- Views: 431
6M.1
A student was given a standard Cu(s)uCu21(aq) half-cell and another half-cell containing an unknown metal M in 1.00 M(NO3)2(aq) and formed the cell M(s)|M+(aq)||Cu2+(aq)|Cu(s). The cell potential was found to be -0.689 V. What is the value of E(M2+/M)? The solutions manual for this problem flips the...
- Sat Feb 29, 2020 2:35 pm
- Forum: Balancing Redox Reactions
- Topic: Test 2
- Replies: 19
- Views: 982
Re: Test 2
It should only be the end of thermodynamics and through electrochemistry. Kinetics should not be on test 2 at all.
- Fri Feb 28, 2020 12:34 am
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Anode vs Cathode
- Replies: 15
- Views: 869
Anode vs Cathode
When given a galvanic cell, how do you determine which is the anode and which is the cathode? Is it just based on the anode being written on the left side and cathode being written on the right?
- Thu Feb 27, 2020 11:31 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: 5.55
- Replies: 3
- Views: 302
5.55
A reaction used in the production of gaseous fuels from coal, which is mainly carbon, is C(s) 1 H2O(g) Δ CO(g) 1 H2(g). (a) Evaluate K At 900 K, given that the standard Gibbs free energies of formation of CO(g) and H2O(g) at 900 K are 2191.28 kJ/mol and 2198.08 kJmol, respectively. (b) A sample of g...
- Thu Feb 27, 2020 6:16 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: 5G.17
- Replies: 2
- Views: 247
5G.17
This question asks us to depict the progress of the reaction I2(g) -> 2I(g) graphically. Can someone explain how to do this?
- Thu Feb 27, 2020 6:14 pm
- Forum: Interesting Applications: Rechargeable Batteries (Cell Phones, Notebooks, Cars), Fuel Cells (Space Shuttle), Photovoltaic Cells (Solar Panels), Electrolysis, Rust
- Topic: pH Electrodes, Corrosion, Etc
- Replies: 2
- Views: 216
pH Electrodes, Corrosion, Etc
I don't recall Dr. Lavelle covering these topics very in depth in class. Will there be problems on them on Test 2?
- Sat Feb 22, 2020 11:17 am
- Forum: Work, Gibbs Free Energy, Cell (Redox) Potentials
- Topic: E cell
- Replies: 6
- Views: 477
E cell
Is the E cell given to us or is it in an appendix or will be have to solve for it?
- Sat Feb 22, 2020 11:15 am
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Pt
- Replies: 7
- Views: 480
Re: Pt
It means platinum and is used as a inert conductor as electrode to transfer electrons. Graphite can also be used the same way.
- Sat Feb 22, 2020 11:13 am
- Forum: Balancing Redox Reactions
- Topic: Charge of oxygen
- Replies: 15
- Views: 756
Re: Charge of oxygen
I believe we can assume that the oxidation number for Oxygen will be -2 and for Hydrogen it will be +1
- Thu Feb 20, 2020 11:52 am
- Forum: Balancing Redox Reactions
- Topic: Balancing Redox Reactions in Basic Solution
- Replies: 4
- Views: 339
Balancing Redox Reactions in Basic Solution
I'm a confused on how to balance a redox reaction in basic solution. Are we supposed to use OH- like we use H+ in a reaction in acidic solution?
- Thu Feb 20, 2020 11:09 am
- Forum: Balancing Redox Reactions
- Topic: Oxidation Numbers
- Replies: 5
- Views: 339
Oxidation Numbers
How do you find oxidation numbers?
- Thu Feb 13, 2020 3:48 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Midterm Curve
- Replies: 45
- Views: 2259
Midterm Curve
Does Lavelle curve his tests at all? Can we expect a curve for the midterm?
- Wed Feb 12, 2020 4:45 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Summary of Units
- Replies: 3
- Views: 222
Summary of Units
q = J
w = J
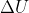 = J
= J
 = J/mol, J/g
= J/mol, J/g
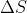 = J/K, J/°C
= J/K, J/°C
 = J
= J
k/Q = no units
Feel free to correct me or add on
w = J
k/Q = no units
Feel free to correct me or add on
- Tue Feb 11, 2020 10:46 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: Balanced Chemical Equations
- Replies: 4
- Views: 437
Balanced Chemical Equations
Why are the balanced chemical equations for this unit have fractional coefficients rather than integers? Would doing the problem with the whole number coefficients change the answer because the ratios would still be the same.
- Tue Feb 11, 2020 8:29 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: Delta U
- Replies: 8
- Views: 579
Delta U
What exactly is 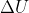 ? I know it means internal energy but I'm not sure what that means.
? I know it means internal energy but I'm not sure what that means.
- Tue Feb 11, 2020 10:53 am
- Forum: Entropy Changes Due to Changes in Volume and Temperature
- Topic: 4F.17
- Replies: 1
- Views: 196
4F.17
Calculate the standard entropy of vaporization of water at 85 C, given that its standard entropy of vaporization at 100 C is 109.0 J/Kmol and the molar heat capacities at constant pressure of liquid water and water vapor are 75.3 J/Kmol and 33.6 J/Kmol, respectively, in this range. I don't know wher...
- Fri Feb 07, 2020 7:49 pm
- Forum: Calculating Work of Expansion
- Topic: Equation for Work
- Replies: 3
- Views: 117
Equation for Work
Is the equation for work always negative even if we are calculating work done on the system?
- Wed Feb 05, 2020 10:39 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: 4D.9
- Replies: 3
- Views: 145
Re: 4D.9
After finding the enthalpy for the reaction, you need to find how much energy is released per mole of TNT. The reaction requires 4 moles of TNT, but since you want the energy per mole of TNT, you divide the reaction enthalpy by 4. Now, all you have to do if multiply this energy by the density of TN...
- Tue Feb 04, 2020 5:56 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: 4D.9
- Replies: 3
- Views: 145
4D.9
The enthalpy of formation of trinitrotoluene (TNT) is -67 kJ/mol, and the density of TNT is 1.65 g/cm^3. In principle, it could be used as rocket fuel, with the gases resulting from its decomposition streaming out of the rocket to give the required thrust. In practice, of course, it would be extreme...
- Tue Feb 04, 2020 10:32 am
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Ideal Gas Heat Capacities
- Replies: 1
- Views: 97
Ideal Gas Heat Capacities
Do we have to memorize the chart on page 266 which has the different coefficients to find the heat capacities of ideal gases? I know we learned (3/2)R and (5/2)R for individual atoms but the book also gives us equations for linear and nonlinear molecules. Homework problem 4C.5 asks about these and i...
- Tue Feb 04, 2020 10:08 am
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Equation for q
- Replies: 4
- Views: 154
Equation for q
When do we use 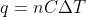 vs.
vs. 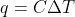
- Tue Feb 04, 2020 10:00 am
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Topics on the Midterm
- Replies: 22
- Views: 1108
Topics on the Midterm
What topics will the midterm cover? Will we have to know all of Thermodynamics as well?
- Thu Jan 30, 2020 6:37 pm
- Forum: Phase Changes & Related Calculations
- Topic: 4A.3
- Replies: 3
- Views: 169
Re: 4A.3
You can find this number in the constants and equations paper. It converts 1 Latm to 101.325 J.
- Thu Jan 30, 2020 6:09 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: 4C.3
- Replies: 1
- Views: 155
4C.3
Calculate the final temperature and the change in enthalpy when 765 J of energy is transferred as heat to 0.820 mol Kr(g) at 298 K and 1.00 atm (a) at constant pressure; (b) at constant volume. Treat the gas as ideal. For part a the solutions manual says q=(5.025 g/83.80 g/mol)(25C - 97.6C)(20.8 J/m...
- Wed Jan 29, 2020 11:28 pm
- Forum: Phase Changes & Related Calculations
- Topic: Units
- Replies: 16
- Views: 845
Re: Units
The temperature is interchangeable because Kelvin and Celsius are to the same scale. So one degree change of Celsius is one degree change in Kelvin and vice versa
- Wed Jan 29, 2020 11:26 pm
- Forum: Phase Changes & Related Calculations
- Topic: Open vs Isolated System
- Replies: 15
- Views: 1318
Re: Open vs Isolated System
Open: can exchange matter and energy
Closed: can exchange only energy
Isolated: can not exchanged anything
Closed: can exchange only energy
Isolated: can not exchanged anything
- Tue Jan 28, 2020 9:43 am
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Textbook Section
- Replies: 2
- Views: 167
Textbook Section
What section of the textbook is reaction enthalpies in? I started reading Focus 4 and didn't see it.
- Sat Jan 25, 2020 11:09 am
- Forum: Phase Changes & Related Calculations
- Topic: Steam Burn
- Replies: 6
- Views: 240
Steam Burn
I am not clear on what Dr. Lavelle was explaining about steam burns. Can someone explain it to me?
- Thu Jan 23, 2020 5:12 pm
- Forum: Non-Equilibrium Conditions & The Reaction Quotient
- Topic: Kw = (Ka)(Kb)
- Replies: 4
- Views: 191
Kw = (Ka)(Kb)
If only Ka or Kb is given and the temperature is not stated, can we assume that it is at 25 Celsius and use 1.0x10^-14 to find the constant we need?
- Thu Jan 23, 2020 12:44 am
- Forum: Non-Equilibrium Conditions & The Reaction Quotient
- Topic: 6D.15 part b
- Replies: 1
- Views: 124
6D.15 part b
Calculate the pH of b) 0.055 M AlCl3. The K value isn't given and is not in the tables in the textbook. How are we supposed to solve this?
- Wed Jan 22, 2020 4:55 pm
- Forum: Non-Equilibrium Conditions & The Reaction Quotient
- Topic: 6D.5
- Replies: 2
- Views: 156
6D.5
Calculate the pH, pOH, and percentage protonation of solute in each of the following aqueous solutions: a) 0.057 M NH3; b) 0.162 M NH2OH; c) 0.35 M (CH3)3N; d) 0.0073 M codeine, given that pKa of its conjugate acid is 8.21. I'm confused on how exactly to do this problem. Are we supposed to have K va...
- Tue Jan 21, 2020 4:49 pm
- Forum: Non-Equilibrium Conditions & The Reaction Quotient
- Topic: 6B.9
- Replies: 2
- Views: 88
6B.9
When the concentrations of H and OH are 1.5 M, isn't the pH and pOH negative?
- Fri Jan 17, 2020 10:21 pm
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Change in Pressure
- Replies: 5
- Views: 180
Change in Pressure
Can someone explain exactly why the reaction shift is dependent on the number of moles on each side of the reaction?
- Thu Jan 16, 2020 11:14 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: 5I.13 c
- Replies: 2
- Views: 158
Re: 5I.13 c
Cl2 is more stable because it has a lower K value. In the reaction Cl2 -> 2Cl, the reactant (Cl2) would be more greatly favored which makes it than the reaction with F2 and thus it is more stable
- Thu Jan 16, 2020 11:12 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Solids and Equilibrium
- Replies: 9
- Views: 303
Re: Solids and Equilibrium
Solids and liquids do not affect because their concentrations do not change.
- Tue Jan 14, 2020 11:39 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: What is K
- Replies: 6
- Views: 240
What is K
What exactly is just K compared to Kc and Kp
- Tue Jan 14, 2020 11:05 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Thermodynamically Stable?
- Replies: 3
- Views: 140
Thermodynamically Stable?
(a) In an experiment, 2.0 mmol Cl2(g) was sealed into a reaction vessel of volume 2.0 L and heated to 1000. K to study its dissociation into Cl atoms. Use the information in Table 5G.2 to calculate the equilibrium composition of the mixture. (b) If 2.0 mmol F2 was placed into the reaction vessel ins...
- Thu Jan 09, 2020 4:15 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Q vs K
- Replies: 7
- Views: 268
Re: Q vs K
Q and K are calculated the same but Q is the value at any point during the reaction while K is the value at equilibrium.
- Thu Jan 09, 2020 4:02 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Why is K unitless?
- Replies: 10
- Views: 628
Why is K unitless?
Why does the equilibrium constant, K, not have units?
- Thu Jan 09, 2020 4:01 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Units
- Replies: 4
- Views: 175
Re: Units
K values do not have units
- Tue Jan 07, 2020 7:43 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Concentrations at Equilibrium
- Replies: 6
- Views: 250
Re: Concentrations at Equilibrium
The concentration of the reactants and products are not necessarily the same; they are just at specific concentrations that allow the reaction to be at equilibrium where both the reaction and reverse reaction occur at an equal rate.
- Tue Jan 07, 2020 7:41 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Expression for K
- Replies: 4
- Views: 189
Re: Expression for K
P means Partial Pressure and the brackets [] mean concentration
- Sat Dec 07, 2019 1:20 pm
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: Naming Coordination Compounds on Final
- Replies: 2
- Views: 157
Naming Coordination Compounds on Final
Do we have to memorize the sheet that Dr. Lavelle provided with how to name ligands for the final?
- Fri Dec 06, 2019 4:24 pm
- Forum: Calculating pH or pOH for Strong & Weak Acids & Bases
- Topic: 6D.11 part e and f
- Replies: 1
- Views: 98
6D.11 part e and f
Decide whether an aqueous solution of each of the following salts has a pH equal to, greater than, or less than 7. If pH < 7 or pH > 7, write a chemical equation to justify your answer. e: AlCl_{3} f: Cu(NO_{3})_{2} I'm not sure how to go about doing these and I don't understand the solution...
- Fri Dec 06, 2019 4:19 pm
- Forum: Calculating pH or pOH for Strong & Weak Acids & Bases
- Topic: 6D.11 part d
- Replies: 1
- Views: 108
6D.11 part d
Why is the pH of KBr neutral but the pH of KF in part c is basic?
- Fri Dec 06, 2019 4:18 pm
- Forum: Calculating pH or pOH for Strong & Weak Acids & Bases
- Topic: 6D.11 part d
- Replies: 1
- Views: 106
6D.11 part d
Why is the pH of KBr neutral but the pH of KF in part c is basic?
- Thu Dec 05, 2019 11:39 pm
- Forum: Lewis Acids & Bases
- Topic: H2PO4-
- Replies: 2
- Views: 119
H2PO4-
Why does 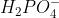 lose another H to become
lose another H to become 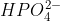 ? Why would it not gain a H to become neutral?
? Why would it not gain a H to become neutral?
- Fri Nov 29, 2019 5:19 pm
- Forum: Naming
- Topic: Coordination Compounds on the Final
- Replies: 4
- Views: 317
Coordination Compounds on the Final
Will there be problems on coordination compounds on the final? I feel we did not cover it much in class and there were very few homework problems on it. What kinds of questions could there be?
- Fri Nov 29, 2019 5:18 pm
- Forum: Student Social/Study Group
- Topic: Post All Chemistry Jokes Here
- Replies: 9651
- Views: 3589409
Re: Post All Chemistry Jokes Here
How often do I tell chemistry jokes??? Periodically
- Fri Nov 29, 2019 5:16 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Studying for the Final [ENDORSED]
- Replies: 11
- Views: 765
Studying for the Final [ENDORSED]
What is the best way to go about studying for the final? Will there be practice finals released?
- Mon Nov 25, 2019 3:03 pm
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: Homework 9C.9 Part C and D
- Replies: 1
- Views: 142
Homework 9C.9 Part C and D
What is "en" and "edta" in part c and d of this problem?
- Mon Nov 25, 2019 2:47 pm
- Forum: Naming
- Topic: Homework 9C.3
- Replies: 2
- Views: 115
Homework 9C.3
I'm having trouble naming compounds. I'm mostly struggling with figuring out how many molecules and what the roman numeral means.
- Fri Nov 22, 2019 2:03 pm
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: Naming Ligands
- Replies: 2
- Views: 258
Naming Ligands
Do we have to memorize all the rules for naming ligands?
- Fri Nov 22, 2019 1:35 pm
- Forum: Hybridization
- Topic: Homework 2.45
- Replies: 1
- Views: 144
Homework 2.45
Part b of this questions asks us to Identify the composition of the bonds and the hybridization of each lone pair—for example, by writing s (H1s,C2sp 2). What do they mean by lone pair? The Lewis structure I drew only has lone pairs around the oxygen for this molecule so do they just want the inform...
- Fri Nov 22, 2019 1:04 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Homework 2.27
- Replies: 1
- Views: 184
Homework 2.27
Part b of this problem asks us to determine if any of the molecules are radicals. I don't think any of the molecules are radicals but I don't have the solutions manual so I am not sure. Are any of the molecules radicals?
- Fri Nov 22, 2019 12:53 pm
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: What are Ligands?
- Replies: 6
- Views: 217
What are Ligands?
I'm a bit confused on what exactly ligands are. Is ligand just a name for a particular segment of a molecule?
- Mon Nov 18, 2019 6:35 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Sigma and Pi Bonds
- Replies: 2
- Views: 266
Sigma and Pi Bonds
What kinds of questions could we get asked and tested on for sigma and pi bonds? There weren't many problems in the homework about it. Will we just be asked how many of each type of bond there are in a molecule?
- Thu Nov 14, 2019 8:12 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Question 2.E.7
- Replies: 3
- Views: 211
Re: Question 2.E.7
Bond angles aren't something that we calculate, we just have to memorize them
- Thu Nov 14, 2019 8:10 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: why are double bonds equally weighted as single ones when drawing models?
- Replies: 10
- Views: 1980
Re: why are double bonds equally weighted as single ones when drawing models?
VSPER model only considers regions of high electron concentration not the actual concentration which is why multiple bonds are treated as a single region of high electron concentration
- Thu Nov 14, 2019 8:05 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Homework 2E.1
- Replies: 4
- Views: 174
Re: Homework 2E.1
You can tell if there are lone pairs based on how the structure is drawn. For part a there has to be lone pairs because of how the other bonds are at an angle which means they are being repelled by a lone pair. For part b there is no obvious repulsion but there can still be lone pairs if there was a...
- Wed Nov 13, 2019 5:31 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Memorization
- Replies: 15
- Views: 1007
Memorization
Will we have to memorize all the different names of the shapes and the bond angles?
- Mon Nov 11, 2019 6:56 pm
- Forum: Interionic and Intermolecular Forces (Ion-Ion, Ion-Dipole, Dipole-Dipole, Dipole-Induced Dipole, Dispersion/Induced Dipole-Induced Dipole/London Forces, Hydrogen Bonding)
- Topic: Homework 3F.5 Part C
- Replies: 2
- Views: 148
Homework 3F.5 Part C
Suggest, giving reasons, which substance in each of the following pairs is likely to have the higher normal melting point: c) CHI3 or CHF3 The solutions manual says CHI3 would have a higher melting point because it has stronger London Dispersion forces but how is the strength of London Dispersion de...
- Sun Nov 10, 2019 2:58 pm
- Forum: Interionic and Intermolecular Forces (Ion-Ion, Ion-Dipole, Dipole-Dipole, Dipole-Induced Dipole, Dispersion/Induced Dipole-Induced Dipole/London Forces, Hydrogen Bonding)
- Topic: How to Draw Dipole Arrows
- Replies: 8
- Views: 2741
How to Draw Dipole Arrows
I'm confused on how to know which way to draw dipole arrows and when to draw them.
- Sun Nov 10, 2019 2:52 pm
- Forum: Interionic and Intermolecular Forces (Ion-Ion, Ion-Dipole, Dipole-Dipole, Dipole-Induced Dipole, Dispersion/Induced Dipole-Induced Dipole/London Forces, Hydrogen Bonding)
- Topic: Test 2
- Replies: 6
- Views: 379
Test 2
Will Test 2 include all the content we've learned thus far or just what we have learned after the midterm?
- Sun Nov 10, 2019 2:49 pm
- Forum: Bond Lengths & Energies
- Topic: Bond Strength
- Replies: 4
- Views: 277
Bond Strength
How exactly is bond strength determined? I know it is dependent on the number of bonds but how do you tell the difference of bond strengths between different atoms?
- Sun Nov 10, 2019 2:45 pm
- Forum: Electronegativity
- Topic: Differences in Electronegativity
- Replies: 5
- Views: 383
Differences in Electronegativity
Do we need to memorize which bonds correspond to which differences in electronegativity?
- Mon Nov 04, 2019 9:34 pm
- Forum: Lewis Structures
- Topic: Double Bonds
- Replies: 3
- Views: 177
Double Bonds
How do you know which atoms to form a double bond with when drawing a lewis structure? For example, why does the double bond form between O and N instead of F and N in the molecule ONF
- Sun Nov 03, 2019 10:41 am
- Forum: Octet Exceptions
- Topic: Valence Electrons for Transition Metals
- Replies: 4
- Views: 519
Valence Electrons for Transition Metals
How do you determine the number of valence electrons for transition metals?
- Sun Nov 03, 2019 10:39 am
- Forum: Formal Charge and Oxidation Numbers
- Topic: Purpose of Formal Charge
- Replies: 6
- Views: 265
Purpose of Formal Charge
What is the purpose of formal charge? I know how to calculate it but what exactly is it for?
- Sun Nov 03, 2019 10:38 am
- Forum: Resonance Structures
- Topic: Drawing Resonance Structures
- Replies: 4
- Views: 295
Drawing Resonance Structures
Is there a quicker way to draw resonance structures or is the only way to just redraw the structure with the different double bonds?
- Wed Oct 30, 2019 6:12 pm
- Forum: Lewis Structures
- Topic: Valence Electrons from Periodic Table
- Replies: 10
- Views: 683
Re: Valence Electrons from Periodic Table
The number of valence electrons is determined by the group number. For groups 13-18, the number of valence electrons is the number in the ones place.
- Wed Oct 30, 2019 5:16 pm
- Forum: Ionic & Covalent Bonds
- Topic: Homework 2A.11
- Replies: 2
- Views: 95
Homework 2A.11
Which M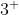 ions (where M is a metal) are predicted to have the following ground-state electron configurations:
ions (where M is a metal) are predicted to have the following ground-state electron configurations:
(a) [Ar]3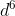
(b) [Ar]3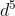
For this problem, why does the solutions manual say that a is Cobalt and b is Iron instead of Iron and Manganese, respectively?
(a) [Ar]3
(b) [Ar]3
For this problem, why does the solutions manual say that a is Cobalt and b is Iron instead of Iron and Manganese, respectively?
- Fri Oct 25, 2019 7:29 pm
- Forum: Electron Configurations for Multi-Electron Atoms
- Topic: Homework Help
- Replies: 5
- Views: 216
Re: Homework Help
For this problem all you really have to look at is whether or not the electrons are put into orbitals properly. There should be one electron in each orbital before pairing all in the same direction and s- orbitals should fill completely before moving on to p- orbitals
- Fri Oct 25, 2019 7:23 pm
- Forum: Electron Configurations for Multi-Electron Atoms
- Topic: HW 1E.23
- Replies: 4
- Views: 260
Re: HW 1E.23
For me the easiest way to find unpaired electrons is to think about how they would be put into orbitals and how you have to put an electron in each orbital before you start pairing. Just figure out the number of valence electrons and put them into orbitals using the arrows.
- Fri Oct 25, 2019 7:02 pm
- Forum: Trends in The Periodic Table
- Topic: Effective Nuclear Charge
- Replies: 2
- Views: 114
Effective Nuclear Charge
What is the equation to determine effective nuclear charge?
- Wed Oct 23, 2019 9:37 pm
- Forum: Trends in The Periodic Table
- Topic: Summary of Periodic Trends
- Replies: 7
- Views: 405
Summary of Periodic Trends
- Atomic radii increase down a group and decrease across a period - Ionic radii increase down a group and decrease across a period, cations are smaller than parent and anions are larger - Ionization energy decreases down a group and increases across a period - Electron affinity is high for elements ...
- Wed Oct 23, 2019 9:29 pm
- Forum: Electron Configurations for Multi-Electron Atoms
- Topic: Order of electron configuration
- Replies: 6
- Views: 262
Re: Order of electron configuration
3d should come before 4s for atoms with an atomic number after 20
- Sat Oct 19, 2019 2:08 pm
- Forum: Photoelectric Effect
- Topic: 1B.25
- Replies: 2
- Views: 160
Re: 1B.25
For this problem you use the equation 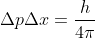 but you break up
but you break up  into (mass of the electron)x
into (mass of the electron)x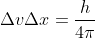
You can then plug in all your known variables and solve for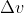
You can then plug in all your known variables and solve for
- Sat Oct 19, 2019 1:55 pm
- Forum: Properties of Light
- Topic: Baler v. Lyman Series
- Replies: 10
- Views: 535
Re: Baler v. Lyman Series
Balmer Series: visible light, n1 = 2
Lyman Series: ultraviolet light, n1 = 1
Lyman Series: ultraviolet light, n1 = 1
- Fri Oct 18, 2019 3:35 pm
- Forum: Heisenberg Indeterminacy (Uncertainty) Equation
- Topic: Homework 1B.25
- Replies: 1
- Views: 142
Homework 1B.25
What is the minimum uncertainty in the speed of an electron confined within a lead atom of diameter 350. pm? model the atom as a one-dimensional box with a length equal to the diameter of the actual atom. I know that I am supposed to use the uncertainty equation for this but I am not sure where to b...
- Wed Oct 16, 2019 3:56 pm
- Forum: Properties of Light
- Topic: 1B #9
- Replies: 5
- Views: 164
Re: 1B #9
32 W = 32 J/s in 2 sec -> 64 J Use the equation E = hc/(wavelength) to find the energy per photon. You should get 4.73 x 10^-19 J/photon Divide 64 J by that number to get the number of photons -- 1.35 x 10^20 photons To find how many moles that is you just divide by Avogadro's number and should get ...
- Wed Oct 16, 2019 3:51 pm
- Forum: Properties of Light
- Topic: Constant for Speed of Light
- Replies: 14
- Views: 608
Re: Constant for Speed of Light
On Lavelle's website there is a link for a pdf with all the constants and equations and the speed of light used in that is 2.99792 × 10^8 m/s so I assume that using 2.998 would be fine
- Sat Oct 12, 2019 8:28 pm
- Forum: Properties of Light
- Topic: 1A.11
- Replies: 2
- Views: 170
Re: 1A.11
The lines are grouped into series based on the lower energy level the electron drops down to and how much energy they’re emitting
- Sat Oct 12, 2019 8:17 pm
- Forum: Properties of Light
- Topic: Equations We’ve Learned So Far
- Replies: 11
- Views: 684
Re: Equations We’ve Learned So Far
Hi Ellis! This is Ariel. Here's a general list from my notes: c = λ ν -- Used to calculate wavelength/frequency E(photon) = h ν -- Used to calculate energy per single photon -- Bohr Frequency Condition: v = ∆E/h; used to calculate frequency E(photon) = Work Function/Threshold Energy + EK EK =(1/2)m...
- Sat Oct 12, 2019 8:13 pm
- Forum: Properties of Light
- Topic: Equations We’ve Learned So Far
- Replies: 11
- Views: 684
Re: Equations We’ve Learned So Far
Ryan Chew 1C wrote:Is there a specific example or equation you're having trouble with? I'm not sure if I can describe all of them.
Maybe for problem 1a.15 I’m confused about the Rydberg equation.
- Sat Oct 12, 2019 7:56 pm
- Forum: Properties of Light
- Topic: Help with Homework 1A.15
- Replies: 3
- Views: 418
Help with Homework 1A.15
1A.15 In the ultraviolet spectrum of atomic hydrogen, a line is observed at 102.6 nm. Determine the values of n for the initial and final energy levels of the electron during the emission of energy that leads to this spectral line. Reading this question, I have no idea where to begin. Is the questio...
- Sat Oct 12, 2019 7:49 pm
- Forum: Properties of Light
- Topic: Equations We’ve Learned So Far
- Replies: 11
- Views: 684
Equations We’ve Learned So Far
I’m having some trouble with all the equations that we’ve learned so far. Can someone list them and explain when we’re supposed to use each one?
- Thu Oct 10, 2019 8:53 pm
- Forum: Properties of Light
- Topic: Electromagnetic Spectrum
- Replies: 5
- Views: 292
Electromagnetic Spectrum
Do we need to memorize the range of wavelengths that each type of radiation have and what order they go in the electromagnetic spectrum. In question 1A 9 in the homework, we are asked to fill out the chart and then determine the type of radiation and events for each line. Are we allowed to refer to ...
- Sun Oct 06, 2019 1:31 pm
- Forum: Empirical & Molecular Formulas
- Topic: Nomenclature for Metals
- Replies: 1
- Views: 175
Nomenclature for Metals
What is the rule for naming formulas with metals? For example, in L39, they ask us to name the oxide and the answer is tin(IV) oxide. Where exactly does the roman numeral come from and how do we determine it?
- Thu Oct 03, 2019 2:13 pm
- Forum: Empirical & Molecular Formulas
- Topic: Homework Problem F1
- Replies: 2
- Views: 191
Re: Homework Problem F1
the way it is written in the solutions manual is a bit confusing. What is really happening is that they are dividing the mass of the element by the total molar mass of the compound to get the fraction/decimal of that element. They then multiply by 100% to convert that decimal into a percentage.
- Thu Oct 03, 2019 2:09 pm
- Forum: Empirical & Molecular Formulas
- Topic: Fundamentals F.25
- Replies: 3
- Views: 221
Re: Fundamentals F.25
To get the empirical formula from the molecular formula, you divide the subscripts by their common factor because the empirical formula is the most simplified version of the ratio of the atoms.
- Wed Oct 02, 2019 3:36 pm
- Forum: Molarity, Solutions, Dilutions
- Topic: How to do E15
- Replies: 4
- Views: 247
Re: How to do E15
First you find the molar mass of (OH)2 by adding together the molar mass of oxygen and hydrogen then multiplying by 2. Then you subtract that from the given molar mass, 74.10, to find the molar mass of the metal. The number you get is 40.08 g/mol which is the molar mass of Calcium - you must refer t...

