Search found 101 matches
- Sat Mar 14, 2020 8:12 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: G at different stages of a run
- Replies: 1
- Views: 223
Re: G at different stages of a run
Yes, we can use Q to determine delta G at any point in a reaction, but it will still be delta G rather than just G because Gibbs free energy refers to amount of energy available to do work, and if there is no change, no work is being done.
- Sat Mar 14, 2020 7:18 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Kw and other constants
- Replies: 9
- Views: 742
Re: Kw and other constants
Kw is the equilibrium constant for the autoprotolysis of water. Because this is equal to the concentrations of OH- times H+, it is also equal to Ka times Kb
- Sat Mar 14, 2020 2:03 pm
- Forum: Entropy Changes Due to Changes in Volume and Temperature
- Topic: UA Practice Final Review Q10
- Replies: 1
- Views: 209
Re: UA Practice Final Review Q10
You can use PV/T due to the ideal gas law, but I believe you would need to multiply by a constant to get the units for entropy, J/K from atm.L/K.
- Sat Mar 14, 2020 1:31 pm
- Forum: Arrhenius Equation, Activation Energies, Catalysts
- Topic: determining a catalyst
- Replies: 5
- Views: 378
Re: determining a catalyst
One defining quality of a catalyst is that it is not used up during the course of a reaction. In the mechanism, it will be includes, but on both sides of a certain step. Also, is the activation energy for a specific reaction is lowered, it is likely there is a catalyst involved in the reaction.
- Sat Mar 14, 2020 12:56 pm
- Forum: Arrhenius Equation, Activation Energies, Catalysts
- Topic: pre-exponential factor
- Replies: 2
- Views: 209
Re: pre-exponential factor
The A is named the pre exponential factor because in the arrhenius equation, it is a constant that is before the exponential part of the equation.
- Sun Mar 08, 2020 9:00 pm
- Forum: General Rate Laws
- Topic: Arrhenius Equation
- Replies: 3
- Views: 337
Re: Arrhenius Equation
The Arrhenius equation allows us to model how temperature affects the rate constant of a certain reaction. If the reaction can be modeled by it, the plot of ln(k) vs. 1/T will be linear.
- Sun Mar 08, 2020 8:54 pm
- Forum: Method of Initial Rates (To Determine n and k)
- Topic: K
- Replies: 11
- Views: 676
Re: K
The equilibrium constant K is equal to the concentration of products divided by the concentration of reactants of a reaction at equilibrium.
- Sun Mar 08, 2020 8:53 pm
- Forum: Reaction Mechanisms, Reaction Profiles
- Topic: Microscopic Reversibility
- Replies: 1
- Views: 156
Re: Microscopic Reversibility
You are correct. Microscopic reversibility is the assumption that the reaction mechanism does not change, but is only reversed when a reaction is reversed.
- Sun Mar 08, 2020 8:10 pm
- Forum: General Rate Laws
- Topic: units
- Replies: 12
- Views: 659
Re: units
The equilibrium constant, K has no units. If you mean rate constant, k, then the units are mol.L-1.s-1 for a zero order reaction, s-1 for a first order reaction, and L.mol-1.s-1 for a second order reaction.
- Sun Mar 08, 2020 8:00 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: galvanic vs electrolytic
- Replies: 12
- Views: 925
Re: galvanic vs electrolytic
A galvanic cell has a positive Eocell value, and therefore the redox reactions and flow of e- is spontaneous. An electrolytic cell has a negative Eocell value, so the flow of electrons requires an external source of current to cause the redox reactions to occur.
- Sun Mar 01, 2020 10:10 pm
- Forum: Interesting Applications: Rechargeable Batteries (Cell Phones, Notebooks, Cars), Fuel Cells (Space Shuttle), Photovoltaic Cells (Solar Panels), Electrolysis, Rust
- Topic: Identifying cathode & anode in electrolytic cells
- Replies: 4
- Views: 385
Re: Identifying cathode & anode in electrolytic cells
Yes I believe your understanding is correct. When calculating E o of a cell, we use the natural E o of reduction values for E o cell = E o R - E o L . This applies even when the cell is unfavorable but you add a current to cause a redox reaction. Reduction always occurs at the cathode and oxidation ...
- Sun Mar 01, 2020 7:44 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: electrodes
- Replies: 5
- Views: 396
Re: electrodes
We always want to use an inert metal such as Platinum because it is not involved in the chemical reaction, only in the transfer of e-.
- Sun Mar 01, 2020 7:42 pm
- Forum: Balancing Redox Reactions
- Topic: basic solution
- Replies: 4
- Views: 334
Re: basic solution
Another way to look at it is using the same process, but adding one step. After we balance using H+, we add an equal amount oh OH- to each side to cancel the charge. Then we combine the OH- and H+ to make H2O and remove excess H2O so that it is only on one side of the equation.
- Sun Mar 01, 2020 7:34 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: How to determine anode and cathode in 6.57?
- Replies: 6
- Views: 458
Re: How to determine anode and cathode in 6.57?
Because you are given Eo for the whole reaction, we can use Eo = Ecathode - Eanode, all we need is the E value for the other half reaction and arrange the equation to give us the given Eo value.
- Sun Mar 01, 2020 7:30 pm
- Forum: Balancing Redox Reactions
- Topic: oxidizing agents
- Replies: 11
- Views: 646
Re: oxidizing agents
The oxidizing agent is the substance that causes oxidation in another substance. The same is true for reducing agents.
- Sun Feb 23, 2020 4:34 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Writing cell diagram
- Replies: 2
- Views: 222
Re: Writing cell diagram
The answer to both questions is that Hg (l) is the anode in this case. With a liquid anode, we include the liquid but since water is our solvent and not involved in either the anode or cathode, we do not include it in the cell diagram.
- Sun Feb 23, 2020 4:29 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: neg vs pos
- Replies: 9
- Views: 760
Re: neg vs pos
If voltage is positive, we have a galvanic cell which creates a flow of e- from the anode to the cathode. If we have negative voltage, we have a flow of e- from cathode to anode, which means we do not have a working cell.
- Sun Feb 23, 2020 4:24 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Identify the state
- Replies: 3
- Views: 264
Re: Identify the state
I believe we know that Co is solid because it is in its natural state without a charge. With Ti 3+ and Ti 2+, thy have a charge, which means they were part of a dissolved salt, and are therefore aqueous. The only time we have ions as a solid are in salts.
- Sun Feb 23, 2020 4:22 pm
- Forum: Balancing Redox Reactions
- Topic: Basic and Acidic Conditions
- Replies: 5
- Views: 423
Re: Basic and Acidic Conditions
Under acidic conditions, we use the H+ ions while balancing the equation. Under basic conditions, we use the H+ initially, but balance the charge with H20 and OH- in order to have the reaction in a basic environment.
- Sun Feb 23, 2020 4:20 pm
- Forum: Balancing Redox Reactions
- Topic: Adding H2O and OH- to Balance
- Replies: 5
- Views: 362
Re: Adding H2O and OH- to Balance
When we balance a redox reaction with alkaline conditions, we initially balance it with H+ ions, but to make the reaction alkaline we add H20 and OH-, balancing the charge of the H+ since there are no H+ ions in an alkaline solution. If it is under acidic conditions, we use the H+ ions.
- Sun Feb 16, 2020 8:22 pm
- Forum: Third Law of Thermodynamics (For a Unique Ground State (W=1): S -> 0 as T -> 0) and Calculations Using Boltzmann Equation for Entropy
- Topic: S = kblnW explanation
- Replies: 4
- Views: 541
Re: S = kblnW explanation
Entropy is equal to the boltzmann constant times the natural log of (number of states)(number of atoms/molecules/objects).
- Sun Feb 16, 2020 8:20 pm
- Forum: Entropy Changes Due to Changes in Volume and Temperature
- Topic: S = 0
- Replies: 21
- Views: 1220
Re: S = 0
Entropy total is equal to 0 when there is a reversible reaction since the reaction is 'at equilibrium' throughout the whole time. Entropy of the surroundings is equal to 0 with an expansion against a vaccum.
- Sun Feb 16, 2020 8:18 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Spontaneous
- Replies: 13
- Views: 832
Re: Spontaneous
If you use the G = H - TS, with a - H and + s, you will have a spontaneous reaction. If you have a +H and -S, the reaction will be non-spontaneous. If both are positive, the reaction will be spontaneous if TS is greater than H. If both are negative, the reaction will be spontaneous if H is greater t...
- Sun Feb 16, 2020 8:05 pm
- Forum: Concepts & Calculations Using Second Law of Thermodynamics
- Topic: How to incorporate 2nd Law of Thermo
- Replies: 3
- Views: 300
Re: How to incorporate 2nd Law of Thermo
Because an irreversible reaction is spontaneous, we know that, according to the 2nd law of thermodynamics, the total entropy in the universe must increase. This tells us that the change in total entropy must be greater than 0.
- Sun Feb 16, 2020 8:01 pm
- Forum: Van't Hoff Equation
- Topic: Van't Hoff Equation
- Replies: 3
- Views: 201
Re: Van't Hoff Equation
The Vant Hoff equation is used to relate the equilibrium constant of a reaction to the changes in enthalpy and temperature. It is used to find how a temperature change affects the equilibrium constant of a reaction.
- Sun Feb 09, 2020 6:30 pm
- Forum: Concepts & Calculations Using Second Law of Thermodynamics
- Topic: Second law equation
- Replies: 3
- Views: 119
Re: Second law equation
I guess my question is more of how does this equation prove that entropy always increases in a system.
- Sun Feb 09, 2020 6:24 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Gibbs Free Energy
- Replies: 2
- Views: 143
Re: Gibbs Free Energy
With U, we only consider it at specific points in time rather than considering how specifically the internal energy of a system has changed.
- Sun Feb 09, 2020 6:21 pm
- Forum: Entropy Changes Due to Changes in Volume and Temperature
- Topic: phase change entropy
- Replies: 3
- Views: 224
Re: phase change entropy
Im pretty sure in this case that q rev = q p because we can consider phase changes to be both reversible and at constant pressure. An easier way to think of this is the transition from ice to water and vice versa under constant pressure because the system in this case is only having heat added to it.
- Sun Feb 09, 2020 6:01 pm
- Forum: Concepts & Calculations Using Second Law of Thermodynamics
- Topic: Evaluating the Stability of a Process
- Replies: 2
- Views: 168
Re: Evaluating the Stability of a Process
Could you please post the exact question number? I would like to confirm, but I believe it would be something like 2HCN --> 2C + H2 + N2.
- Sun Feb 09, 2020 5:55 pm
- Forum: Concepts & Calculations Using Second Law of Thermodynamics
- Topic: Second law equation
- Replies: 3
- Views: 119
Second law equation
Hello everyone
Can someone explain the second law of thermodynamics in relation to the equation (delta)S = qrev / T? I am confused as to what this equation means in a conceptual sense.
Can someone explain the second law of thermodynamics in relation to the equation (delta)S = qrev / T? I am confused as to what this equation means in a conceptual sense.
- Sun Feb 09, 2020 5:47 pm
- Forum: Entropy Changes Due to Changes in Volume and Temperature
- Topic: 4I.5
- Replies: 4
- Views: 164
Re: 4I.5
I think the reason you assume constant volume is because it is in an insulated container which would be completely sealed and not able to expand, hence no change in volume.
- Sun Feb 02, 2020 3:41 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Difference between delta H and q
- Replies: 2
- Views: 112
Re: Difference between delta H and q
It does not matter because (delta)H=qp as long as the system is under constant pressure.
- Sun Feb 02, 2020 3:01 pm
- Forum: Thermodynamic Systems (Open, Closed, Isolated)
- Topic: Closed Systems
- Replies: 14
- Views: 960
Re: Closed Systems
In a closed system, you can change the energy by adding heat and/or doing work on the system. I am less certain with the isolated system, but I am pretty sure there should be no net change in the total energy of the system.
- Sun Feb 02, 2020 2:48 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: universe is an isolated system
- Replies: 4
- Views: 172
Re: universe is an isolated system
When we discuss the thermodynamic universe, we are referring to a system and its surroundings. If energy is transferred from a system to the surroundings, it remains in the universe. Energy cannot transfer from the universe or into the universe, because then there would just be more surroundings to ...
- Sun Feb 02, 2020 2:41 pm
- Forum: Environment, Fossil Fuels, Alternative Fuels
- Topic: which section is this info in??
- Replies: 5
- Views: 909
Re: which section is this info in??
Check Dr. Lavelle's website under outlines. The ones we are going through now are thermochemistry and thermodynamics.
- Sun Feb 02, 2020 2:38 pm
- Forum: Calculating Work of Expansion
- Topic: work integral
- Replies: 6
- Views: 253
Re: work integral
We can use -p(delta)v when we have constant pressure, because we can take of the p from the integral since it is a constant. In some way, we are always using the integral, but with constant pressure we can simplify it to make life easier. In the case of pressure not being constant, we must use the i...
- Sun Jan 26, 2020 12:44 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: 4B.3
- Replies: 1
- Views: 83
Re: 4B.3
My textbook says the answer should be 4.90x102 which is the answer you have. I believe your answer is correct.
- Sun Jan 26, 2020 12:41 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: standard reaction enthalpy
- Replies: 1
- Views: 65
Re: standard reaction enthalpy
When it states they are in the same state, that means there are no phase changes in the chemical reaction. The standard reaction enthalpy is the enthalpy of a specific reaction with standard conditions, such as 25 degrees C, 1 atm, and all products and reactants are in the same phase.
- Sun Jan 26, 2020 12:36 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Br2(l) --> 2Br(g)
- Replies: 2
- Views: 560
Re: Br2(l) --> 2Br(g)
We know to use the phase change from a liquid to gas, Hvap because that is what is demonstrated in the equation. We then use the H for breaking bonds because the Br2 becomes 2Br.
- Sat Jan 25, 2020 4:39 pm
- Forum: Phase Changes & Related Calculations
- Topic: Bond Enthalpies
- Replies: 5
- Views: 130
Re: Bond Enthalpies
Using bond enthalpies is inaccurate because all enthalpies for everything except diatomic molecules are calculated averages, and not measured.
- Sat Jan 25, 2020 4:30 pm
- Forum: Phase Changes & Related Calculations
- Topic: Standard Enthalpy of Formation
- Replies: 2
- Views: 113
Re: Standard Enthalpy of Formation
The standard enthalpy of formation refers to the formation of a substance from it's most basic elemental components in their standard states. The case when it is 0 is when we consider elements being formed from their basic elements. The example in class was O 2 becoming O 2 . Because there is no cha...
- Sun Jan 19, 2020 7:17 pm
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Exo/Endo-thermic Rxns
- Replies: 5
- Views: 304
Re: Exo/Endo-thermic Rxns
I'm not quite sure what you are exactly asking, but the endothermic/exothermic reactions refers to the reaction requiring or releasing heat. Endothermic reactions require heat to occur, while exothermic reactions release heat as a product.
- Sun Jan 19, 2020 7:12 pm
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Henderson Hasselbalch Equation
- Replies: 3
- Views: 258
Re: Henderson Hasselbalch Equation
I am fairly certain that we will not be using that equation in this class, and if we do, I'm sure Dr. Lavelle will explain/derive it in lecture. I have never heard of that equation until it was mentioned by a student in class.
- Sun Jan 19, 2020 7:09 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: When do we use the equilibrium sign?
- Replies: 7
- Views: 323
Re: When do we use the equilibrium sign?
Technically, we can use the equilibrium sign in almost every chemical reaction as there usually is a point of equilibrium. The reason we do not use it for strong acids and bases is because the concentration of reactant is so small, it is basically 0.
- Sun Jan 19, 2020 7:06 pm
- Forum: Calculating pH or pOH for Strong & Weak Acids & Bases
- Topic: 5I.33
- Replies: 1
- Views: 189
Re: 5I.33
To start off, you want to change all gram measurements into moles by dividing by the grams/mole of each substance because we are dealing with equilibrium. From the equation, we know that 2 moles of NH 3 are formed per mole of CO 2 formed, so we multiply our moles of CO 2 by 2 to get our moles of NH ...
- Sun Jan 19, 2020 6:59 pm
- Forum: Calculating pH or pOH for Strong & Weak Acids & Bases
- Topic: Molar concentration of acids & bases
- Replies: 8
- Views: 409
Re: Molar concentration of acids & bases
If you are asked specifically for molar concentrations, you would give the moles/Liter rather than the pH or pOH.
- Sun Jan 12, 2020 3:08 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: 5I.13 part c
- Replies: 2
- Views: 108
Re: 5I.13 part c
In the description of the answer, it states that you can compare the equilibrium constants of the dissociation of F2 and Cl2 at a certain temperature. Because the K of Cl2 is lower, it dissociates less and is therefore more thermodimanically stable.
- Sun Jan 12, 2020 2:19 pm
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: 5J:5d
- Replies: 2
- Views: 116
Re: 5J:1d
Because it is an increase in pressure by compression, the moles of each gas does not change, but the volume does. The reaction shifts right for a little bit, but the overall K value does not change.
- Sun Jan 12, 2020 1:51 pm
- Forum: Non-Equilibrium Conditions & The Reaction Quotient
- Topic: Partial Pressures
- Replies: 4
- Views: 229
Re: Partial Pressures
You can use partial pressures for ICE tables if you are given them and Kp. We also use partial pressures in the ideal gas equation and in calculation Kp.
- Sun Jan 12, 2020 1:48 pm
- Forum: Non-Equilibrium Conditions & The Reaction Quotient
- Topic: Ice tables for partial pressures
- Replies: 4
- Views: 198
Re: Ice tables for partial pressures
I believe we can because the Ice tables are used to help with the calculations of concentrations, and if we use Kp, we can, and have to, use partial pressures in the calculation.
- Sun Jan 12, 2020 1:37 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: K = 1
- Replies: 2
- Views: 71
Re: K = 1
K = 1 is rare and difficult to achieve because it requires that the concentrations of both the products and reactants are equal to each other, which is fairly rare in most actual reactions.
- Sat Dec 07, 2019 1:15 pm
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: Chelating ligands
- Replies: 1
- Views: 150
Re: Chelating ligands
When a ligand binds to a central cation, it is chelating. Chelating is just another term for bonding used specifically for ligands.
- Sat Dec 07, 2019 1:13 pm
- Forum: Biological Examples
- Topic: 4 Myoglobin=Hemoglobin
- Replies: 1
- Views: 114
Re: 4 Myoglobin=Hemoglobin
Myoglobin is a different compound from Hemoglobin. In Hemoglobin, four Heme groups rather than myoglobin groups.
- Fri Dec 06, 2019 7:53 pm
- Forum: Polyprotic Acids & Bases
- Topic: polyprotic acids and bases
- Replies: 2
- Views: 1827
Re: polyprotic acids and bases
An acid or base is polyprotic if it can give off or accept multiple protons, respectively. NH4+ is not polyprotic because once it donates one proton, the molecule becomes stable to the point that it will not donate another proton.
- Fri Dec 06, 2019 7:46 pm
- Forum: Bronsted Acids & Bases
- Topic: bronsted base and acid
- Replies: 4
- Views: 416
Re: bronsted base and acid
Something is a Bronsted acid if it gives off a proton to water in solution. A bronsted base removes a proton from water in solution. A lewis acid accepts an electron pair, while a lewis base donates an electron pair.
- Fri Dec 06, 2019 6:28 pm
- Forum: Naming
- Topic: Coordination Number
- Replies: 3
- Views: 309
Re: Coordination Number
The coordination number is the number of ligands around a transition metal cation in a coordination compound. In this case, there are 6 CN- groups around one Fe2+ cation, so the coordination number is 6.
- Thu Dec 05, 2019 8:31 pm
- Forum: Sigma & Pi Bonds
- Topic: Delocalized bonnding
- Replies: 2
- Views: 269
Re: Delocalized bonnding
A molecule with resonance is a molecule that has delocalized bonding. Also, for acids and bases, if a central atom is attached to highly electronegative atoms, the delocalization of the charge will increase.
- Thu Dec 05, 2019 8:20 pm
- Forum: Biological Examples
- Topic: Coordination Number vs Oxidation State
- Replies: 2
- Views: 210
Re: Coordination Number vs Oxidation State
The coordination number refers to the number of coordinate bonds surrounding a cation, while the oxidation number refers to the charge of a transition metal.
- Thu Dec 05, 2019 6:07 pm
- Forum: Bronsted Acids & Bases
- Topic: Difference between bronsted and conjugate
- Replies: 2
- Views: 149
Re: Difference between bronsted and conjugate
Bronsted acids donate protons or lose H+ ions, and bronsted bases accept protons or gain H+ ions. Conjugate acids and bases are bronsted acids or bases, but correlate with a bronsted acid or base. A bronsted acid loses an H+ to become a conjugate base. A bronsted base gains an H+ to become a conjuga...
- Sun Dec 01, 2019 6:17 pm
- Forum: Bronsted Acids & Bases
- Topic: Acidic Oxide
- Replies: 1
- Views: 151
Re: Acidic Oxide
Are the molecules in this equation in the aqueous state? If so, the NaOH acts as a base, and C02 acts as an acid, combining to create a complex salt and water. NaOH reacts with water to produce OH- and CO2 reacts to form a bronsted acid, which donates a proton to OH-, creating H20.
- Sun Dec 01, 2019 6:13 pm
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: Coordinate covalent bonds
- Replies: 1
- Views: 68
Re: Coordinate covalent bonds
Coordinate covalent bonds occur when a covalent bond is created by the sharing of 2e- from a single atom with another atom without lone pair e-. As this is a covalent bond, it has the properties of a covalent bond.
- Sun Dec 01, 2019 6:10 pm
- Forum: Naming
- Topic: Naming coordination compounds
- Replies: 5
- Views: 393
Re: Naming coordination compounds
The roman numeral corresponds with the positive charge, or oxidation state, of the metal in a coordination compound. They should always be included in the name right after the metal.
- Sun Dec 01, 2019 6:08 pm
- Forum: Conjugate Acids & Bases
- Topic: Acids and Bases
- Replies: 3
- Views: 295
Re: Acids and Bases
Bronsted acids are proton, or H+ donators. Bronsted bases are proton acceptors. Conjugate acids are bronsted bases that have accepted a proton. Conjugate bases are bronsted acids that have donated a proton. Lewis acids are electron acceptors, while lewis bases are electron donators.
- Sun Dec 01, 2019 6:05 pm
- Forum: Calculating pH or pOH for Strong & Weak Acids & Bases
- Topic: pH calcuations
- Replies: 8
- Views: 477
Re: pH calcuations
Other than molarity calculations we did ad the beginning of the quarter, we have not done any other calculations with pH besides the ones you have mentions, so I assume so.
- Mon Nov 25, 2019 12:25 am
- Forum: Bond Lengths & Energies
- Topic: Hydrogen Bonding and Dispersion
- Replies: 5
- Views: 375
Re: Hydrogen Bonding and Dispersion
Hydrogen bonding occurs between hydrogen bonded to a F, O, or N and a lone pair of electrons on one of those molecules. London dispersion forces occur between all atoms/molecules and are the same as induced dipole-induced dipole forces in which random e- movement causes areas that are partially char...
- Sun Nov 17, 2019 11:02 pm
- Forum: Ionic & Covalent Bonds
- Topic: Hydrogen Bonds
- Replies: 2
- Views: 192
Re: Hydrogen Bonds
Hydrogen bonds do not strengthen covalent bonds. Covalent bonds are between atoms, while hydrogen bonds are between molecules. They increase the boiling point of substances because they are another bond that must be broken to turn a substance into a gas from a liquid. The most notable interaction is...
- Sun Nov 17, 2019 10:56 pm
- Forum: Interionic and Intermolecular Forces (Ion-Ion, Ion-Dipole, Dipole-Dipole, Dipole-Induced Dipole, Dispersion/Induced Dipole-Induced Dipole/London Forces, Hydrogen Bonding)
- Topic: can someone explain ion-dipole?
- Replies: 3
- Views: 237
Re: can someone explain ion-dipole?
Ion-dipole occurs when an ion, such as Na+ interacts with a polar molecule, such as water. The partially negative oxygen will interact with the Na+ ion. Induced dipole-induced dipole occurs between all atoms/molecules and happens when random electron movement causes partially charged regions of the ...
- Sun Nov 17, 2019 10:54 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Textbook question 2.63 part b
- Replies: 1
- Views: 109
Re: Textbook question 2.63 part b
The lone pair e- for O are not drawn, but there are 2 lone pairs because O needs an octet. This means O will have 4 bonding regions, and thus a tetrahedral shape with bond angles close to 109.5 degrees.
- Sun Nov 17, 2019 10:50 pm
- Forum: Student Social/Study Group
- Topic: Bond Angles
- Replies: 6
- Views: 523
Re: Bond Angles
I don't think we need to know the specific bond angles, but we should know NH 3 bond angles are more than H 2 0 angles because we can look at how many bonding pairs there are versus how many lone pairs there are. The bond angles in H 2 0 are less than NH 3 because H 2 0 has more lone pair electrons,...
- Sun Nov 17, 2019 10:47 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Lone vs. Bonding Pair
- Replies: 6
- Views: 388
Re: Lone vs. Bonding Pair
Lone pairs are not bound to another atom, so the repulsion forces are not weakened by protons in another atom.
- Sun Nov 10, 2019 9:36 pm
- Forum: Dipole Moments
- Topic: London Interaction Strength
- Replies: 3
- Views: 189
Re: London Interaction Strength
The strength of London forces are dependent on the same factors as dipole-dipole bonds. If two molecules are more rod shaped, the bond will be stronger than if they were spherical. The closer the molecules are, the stronger the bond will be.
- Sun Nov 10, 2019 9:34 pm
- Forum: Electronegativity
- Topic: dipole moments
- Replies: 7
- Views: 341
Re: dipole moments
Yes, larger differences in electronegativity cause polar covalent bonding. However, certain molecular shapes, such as tetrahedral, tend to not have a dipole moment because the dipole moment is actually a vector sum between all polar bonds in the molecule.
- Sun Nov 10, 2019 9:26 pm
- Forum: Interionic and Intermolecular Forces (Ion-Ion, Ion-Dipole, Dipole-Dipole, Dipole-Induced Dipole, Dispersion/Induced Dipole-Induced Dipole/London Forces, Hydrogen Bonding)
- Topic: Fluctuating electron distribution
- Replies: 1
- Views: 134
Re: Fluctuating electron distribution
The fluctuation in electron distribution causes certain regions of an atom to become partially charged. This partial charge in one atom has the ability to cause the same effect in a nearby atom due to the repulsion forces of two charges of the same kind. Another name for this random fluctuations of ...
- Sun Nov 10, 2019 8:32 pm
- Forum: Interionic and Intermolecular Forces (Ion-Ion, Ion-Dipole, Dipole-Dipole, Dipole-Induced Dipole, Dispersion/Induced Dipole-Induced Dipole/London Forces, Hydrogen Bonding)
- Topic: Fluctuating Dipoles
- Replies: 5
- Views: 251
Re: Fluctuating Dipoles
Fluctuating dipoles and electron fluctuation refers to the random movement of electrons around an atom/molecule. This allows regions to become partially charged, which in turn causes regions of another molecule/atom to become partially charged. This causes attraction known as van der waals/induced d...
- Sun Nov 10, 2019 8:27 pm
- Forum: Octet Exceptions
- Topic: Valence Electrons for Transition Metals
- Replies: 4
- Views: 526
Re: Valence Electrons for Transition Metals
The same way you would any other atom. It helps to write out the e- configurations in order to do this, and the valence e- will be the number of e- in the outermost layer. Most transition metals have 2 valence e-. Is this because the d orbital tends to fill either halfway or completely full? Most h...
- Sun Nov 03, 2019 4:46 pm
- Forum: Ionic & Covalent Bonds
- Topic: Drawing Ionic Compunds
- Replies: 7
- Views: 589
Re: Drawing Ionic Compunds
If it is a true ionic bond, yes, because drawing a bond represents a covalent bond.
- Sun Nov 03, 2019 4:45 pm
- Forum: Dipole Moments
- Topic: drawing dipole moments
- Replies: 5
- Views: 679
Re: drawing dipole moments
A dipole moment occurs in covalent bonds between atoms of differing electronegativities. To draw a dipole moment, draw an arrow from the lower electronegative atom towards the higher electronegative atom. The vector sum of these arrows will be the overall dipole moment for the molecule.
- Sun Nov 03, 2019 4:38 pm
- Forum: Lewis Structures
- Topic: Bonds
- Replies: 1
- Views: 141
Re: Bonds
Atoms can have more than 8 e- when they have subshells greater than s or p. Atoms can use these orbitals to bond even if they do not have e- in them to begin with, but are in the third row of the periodic table or lower.
- Sun Nov 03, 2019 11:46 am
- Forum: Ionic & Covalent Bonds
- Topic: Drawing Ionic Compunds
- Replies: 7
- Views: 589
Re: Drawing Ionic Compunds
To draw ionic compounds, draw the lewis structures of each ion individually, and label the charge of each and put them in brackets.
- Sun Nov 03, 2019 11:19 am
- Forum: Octet Exceptions
- Topic: Valence Electrons for Transition Metals
- Replies: 4
- Views: 526
Re: Valence Electrons for Transition Metals
The same way you would any other atom. It helps to write out the e- configurations in order to do this, and the valence e- will be the number of e- in the outermost layer. Most transition metals have 2 valence e-.
- Sun Oct 27, 2019 7:43 pm
- Forum: Octet Exceptions
- Topic: Exceptions
- Replies: 3
- Views: 158
Re: Exceptions
H, He, Li, and Be all want to fill only the 1s orbitals, only wanting 2 e- total. As for the atom with the least ionization energy being the central atom, the lower the ionization energy, the more the atom will hold on to its e-. This means that it will be more likely to bond with multiple atoms and...
- Sun Oct 27, 2019 7:36 pm
- Forum: Bond Lengths & Energies
- Topic: Single bond vs double bond
- Replies: 14
- Views: 917
Re: Single bond vs double bond
These numbers represent the observed length of each bond. The delocalization of e- means that the e- is not around one specific atom, but around the entire molecule as a whole.
- Sun Oct 27, 2019 7:30 pm
- Forum: Resonance Structures
- Topic: Lewis Structures for Resonance
- Replies: 4
- Views: 108
Re: Lewis Structures for Resonance
No, for certain atoms with d-orbitals, there can be more than an octet surrounding a specific atom. The example we went through in class that shows this is the sulfate ion, SO42-.
- Sun Oct 27, 2019 5:07 pm
- Forum: Quantum Numbers and The H-Atom
- Topic: determining the number of orbitals
- Replies: 7
- Views: 332
Re: determining the number of orbitals
The Quantum number that describes the orbitals in a subshell is ml. The formula for this is l, (l-1),..., -l.
- Sun Oct 27, 2019 4:59 pm
- Forum: Octet Exceptions
- Topic: Octet rule exception
- Replies: 2
- Views: 138
Re: Octet rule exception
Yes, I believe that all atoms with a d-orbital or higher have the ability to be an exception to the octet rule. These orbitals do allow for more than 8 electrons around a singular atom while drawing Lewis structures.
- Sun Oct 20, 2019 10:12 pm
- Forum: Electron Configurations for Multi-Electron Atoms
- Topic: Writing Electron configurations
- Replies: 2
- Views: 129
Re: Writing Electron configurations
I believe you always put the closest noble gas with atomic number less than the atom you are referring to in brackets to save time and space while writing e- configurations.
- Sun Oct 20, 2019 10:10 pm
- Forum: Heisenberg Indeterminacy (Uncertainty) Equation
- Topic: Heisenberg In 1 Dimension
- Replies: 2
- Views: 129
Heisenberg In 1 Dimension
Hello everybody,
Is it safe to assume that for the purposes of this class, we will only be referring to the Heisenberg Indeterminacy equation in one dimension for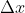 ? Would there be a case in which we used this equation in three dimensions (x,y,z)?
? Would there be a case in which we used this equation in three dimensions (x,y,z)?
Is it safe to assume that for the purposes of this class, we will only be referring to the Heisenberg Indeterminacy equation in one dimension for
- Sun Oct 20, 2019 10:01 pm
- Forum: Wave Functions and s-, p-, d-, f- Orbitals
- Topic: Exceptions
- Replies: 2
- Views: 119
Re: Exceptions
I assume you are asking why these electron configurations are they way they are. I understand that the electron configurations drop a 4s e- because it is easier (takes less energy) to have more stable shells such as 3d^5 or 3d^10. This is the example for Cr and Cu, but it also applies further down t...
- Sun Oct 20, 2019 9:54 pm
- Forum: Electron Configurations for Multi-Electron Atoms
- Topic: Electron Configuration Rules
- Replies: 3
- Views: 157
Re: Electron Configuration Rules
The way I understand the exceptions is that it takes more energy to have 2 e- in the 4d orbital than to have one in each sublevel evenly distributed (hence Cr: [Ar] 3d^5 4s^1). This also applies for copper in which the 3d shell is full.
- Sun Oct 20, 2019 9:47 pm
- Forum: Wave Functions and s-, p-, d-, f- Orbitals
- Topic: Planes
- Replies: 3
- Views: 115
Re: Planes
If an electron is in a plane, it is aligned with a certain plane (x,y,z). I'm not quite sure what you are asking for, but I hope this helps.
- Sun Oct 13, 2019 9:38 pm
- Forum: *Black Body Radiation
- Topic: 1B.3
- Replies: 4
- Views: 312
Re: 1B.3
Electron diffraction has to deal with electrons having wavelike properties, and atomic spectra deals with light as a wave and quantized levels of electron energy. I am not quite sure about black body radiation though.
- Sun Oct 13, 2019 9:36 pm
- Forum: Properties of Light
- Topic: Reading entire chapters before class?
- Replies: 4
- Views: 232
Re: Reading entire chapters before class?
Although it would be helpful to read all chapters in the quantum world, I believe you would be okay to read the chapters that are associated with homework problems. this will not only help you with doing the homework, but also gain a better conceptual understanding of the quantum world for tests, etc.
- Sun Oct 13, 2019 9:32 pm
- Forum: Heisenberg Indeterminacy (Uncertainty) Equation
- Topic: Indeterminancy In Class
- Replies: 7
- Views: 256
Re: Indeterminancy In Class
No, we did not discuss it in depth in class, only mentioned it briefly. I believe we will be discussing it tomorrow. You can double check the outlines on the website to be sure.
- Sun Oct 13, 2019 9:30 pm
- Forum: *Black Body Radiation
- Topic: 1B.3
- Replies: 4
- Views: 312
Re: 1B.3
I believe the correct answer would be the photoelectric effect for a few reasons. The first being that only certain frequencies of light are able to eject electrons from the metal. If light only had wavelike properties, the total amount of energy (including amplitude and frequency) would eject elect...
- Sun Oct 13, 2019 9:18 pm
- Forum: Properties of Electrons
- Topic: Help on 1E.17
- Replies: 2
- Views: 95
Re: Help on 1E.17
I believe it will depend on the number of electrons in the orbitals. If there are two in a specific orbital and one is removed, the other will remain in that orbital. If there is only one in a certain orbital and it is removed, the next electron will be housed in the orbital before. Also, I believe ...
- Sun Oct 13, 2019 9:01 pm
- Forum: Properties of Electrons
- Topic: Change in electron energy
- Replies: 2
- Views: 117
Re: Change in electron energy
I believe the reason for this is back in the idea that energy cannot be created or destroyed. When an electron goes from a higher to a lower state, there has been a decrease of energy, but that energy must go somewhere. In the case of electrons it is released as light.
- Sun Oct 06, 2019 1:49 pm
- Forum: Properties of Light
- Topic: Photons
- Replies: 4
- Views: 122
Re: Photons
So they are a type of particle though?
- Sun Oct 06, 2019 1:04 pm
- Forum: SI Units, Unit Conversions
- Topic: Homework Vs. Test
- Replies: 3
- Views: 180
Homework Vs. Test
Hi everyone,
I noticed that on certain homework problems, such as E.9, we are not given the molecular formula of a compound, but rather the name of the compound. Does anybody know if this will be the case on tests, or will we be given the formula as well as the name?
I noticed that on certain homework problems, such as E.9, we are not given the molecular formula of a compound, but rather the name of the compound. Does anybody know if this will be the case on tests, or will we be given the formula as well as the name?
- Sun Oct 06, 2019 1:00 pm
- Forum: SI Units, Unit Conversions
- Topic: homework E9
- Replies: 1
- Views: 114
Re: homework E9
I could be wrong, but I believe that units are not used because of the convention for atoms and formula units. Because there is no unit besides "atom(s)" or "formula unit(s)" that could be used, you can leave them off. I'm not 100% sure about that though.
- Sun Oct 06, 2019 12:51 pm
- Forum: Properties of Light
- Topic: Photons
- Replies: 4
- Views: 122
Photons
Hi everybody,
I have a few questions about photons that came up during lecture last week. Are photons a type of particle like electrons/neutrons/protons? Or are they what we use in order to describe light with the properties of particles? I'm just getting a little confused...
I have a few questions about photons that came up during lecture last week. Are photons a type of particle like electrons/neutrons/protons? Or are they what we use in order to describe light with the properties of particles? I'm just getting a little confused...

