Test 2 and the corresponding answer keys can be found here.
viewtopic.php?f=160&t=62122&p=237630&sid=fbd87ea7b2442d1db0b2bd7a14b04e68#p237630
Search found 102 matches
- Mon Mar 16, 2020 2:22 pm
- Forum: Balancing Redox Reactions
- Topic: Test 2 Return
- Replies: 20
- Views: 1208
- Wed Mar 11, 2020 2:46 am
- Forum: Reaction Mechanisms, Reaction Profiles
- Topic: Bottle Neck Effect
- Replies: 4
- Views: 379
Re: Bottle Neck Effect
The first step is fast and the second step is slow.
- Tue Mar 10, 2020 8:47 am
- Forum: Zero Order Reactions
- Topic: Zero Order
- Replies: 8
- Views: 516
Re: Zero Order
Are zero order reactions common?
- Tue Mar 10, 2020 8:47 am
- Forum: General Rate Laws
- Topic: 7A.3
- Replies: 6
- Views: 467
Re: 7A.3
Since the question is asking about the rate of consumption, it is already taking into account that oxygen is being used up. Therefore the answer is meant to be positive in terms of consumption rate.
- Mon Mar 09, 2020 10:53 am
- Forum: Arrhenius Equation, Activation Energies, Catalysts
- Topic: Derivation of Arrhenius
- Replies: 2
- Views: 708
Derivation of Arrhenius
How do you derive this equation from the Arrhenius Equation?
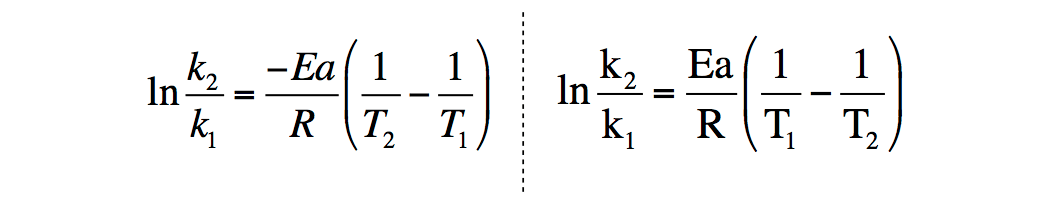
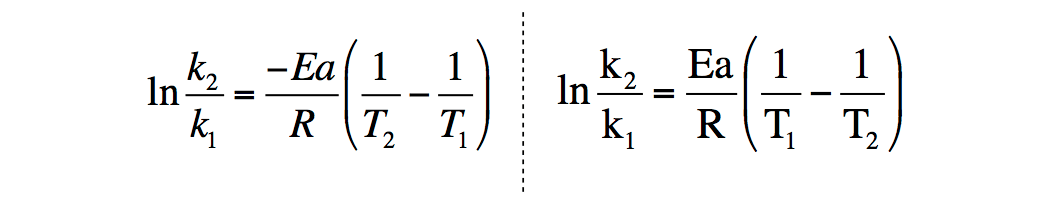
- Mon Mar 09, 2020 10:49 am
- Forum: Reaction Mechanisms, Reaction Profiles
- Topic: Rate laws for reaction mechanisms
- Replies: 3
- Views: 304
Re: Rate laws for reaction mechanisms
I also had the same question. We did not do any practice problems in class and it seems particularly complex.
- Wed Mar 04, 2020 3:35 pm
- Forum: Reaction Mechanisms, Reaction Profiles
- Topic: Rate-Determining Step
- Replies: 4
- Views: 410
Re: Rate-Determining Step
You can determine the rate limiting reaction from the rate law.
- Tue Mar 03, 2020 10:25 am
- Forum: General Rate Laws
- Topic: rate law definition
- Replies: 4
- Views: 394
Re: rate law definition
The rate law tells you the instantaneous reaction rate in terms of the concentration. Each reaction has a unique rate law which is specific to its reactants.
- Tue Mar 03, 2020 10:22 am
- Forum: First Order Reactions
- Topic: first order graphs
- Replies: 2
- Views: 266
Re: first order graphs
For first order reactions, it is good to know how the graph looks in terms of [A] and ln[A]. Below is general trend for first order reactions in both situations. https://saylordotorg.github.io/text_general-chemistry-principles-patterns-and-applications-v1.0/section_18/0fd0e9359ab66f04b9ee0e414d2d9a9...
- Mon Mar 02, 2020 10:26 am
- Forum: Method of Initial Rates (To Determine n and k)
- Topic: k
- Replies: 10
- Views: 609
Re: k
Capital K is the equilibrium constant while lower case k is the rate constant for kinetics.
- Mon Mar 02, 2020 10:25 am
- Forum: General Rate Laws
- Topic: Half Lives
- Replies: 3
- Views: 279
Re: Half Lives
Yes, you need to use half life. Since 1/4 is (1/2)^2, to have your final concentration be a fourth of the initial, it would take two half lives. Correspondingly, it would take 3 half lives to to get 1/8 of the initial.
- Thu Feb 27, 2020 9:33 am
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: E cell
- Replies: 2
- Views: 211
Re: E cell
You can use the equation, E = Eo - RT ln Q, to find the electric potential under non standard conditions.
- Wed Feb 26, 2020 10:06 am
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: 6M7
- Replies: 3
- Views: 238
Re: 6M7
Use the value that corresponds with reduction potential for the substance in a solid state.
- Wed Feb 26, 2020 10:05 am
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: determining unknown quantity in cell
- Replies: 2
- Views: 201
Re: determining unknown quantity in cell
For the units to cancel out correctly, you have to use R = 8.314 J/(mol*K). Whenever you need to decide what R to use, always check the units of the other values given.
- Mon Feb 24, 2020 7:43 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Concentration Cells
- Replies: 5
- Views: 381
Re: Concentration Cells
In the example during class, a porous membrane separated the two sides of the cell. However, only then anion was able to pass this membrane.
- Mon Feb 24, 2020 11:37 am
- Forum: Balancing Redox Reactions
- Topic: 6K.3 part d
- Replies: 3
- Views: 273
Re: 6K.3 part d
I believe that the product is supposed to be Cl- not Cl2.
- Wed Feb 19, 2020 10:24 am
- Forum: Balancing Redox Reactions
- Topic: What is being reduced/oxidized in this rxn?
- Replies: 4
- Views: 372
Re: What is being reduced/oxidized in this rxn?
I believe it is an error in the question. It should be Cl2 --> 2Cl-. This will show the change in oxidation state.
- Wed Feb 19, 2020 10:21 am
- Forum: Balancing Redox Reactions
- Topic: Oxidation Number Rules
- Replies: 7
- Views: 510
Re: Oxidation Number Rules
Here is a chart that can help with oxidation numbers.


- Tue Feb 18, 2020 3:41 pm
- Forum: Balancing Redox Reactions
- Topic: Finding charge
- Replies: 2
- Views: 255
Re: Finding charge
You can also find the charge by looking at the charge of the compound as a whole. For example if you have MnO4-. The total net charge for the compound is -1 and we know that oxygen has a oxidation number of -2. Since there are 4 oxygens we know that the total charge that comes form the oxygens are -...
- Mon Feb 17, 2020 12:58 pm
- Forum: Van't Hoff Equation
- Topic: When to Use Vant Hoff
- Replies: 5
- Views: 404
Re: When to Use Vant Hoff
You either have to be given the standard enthalpy or find it using a table of values to solve for the delta H.
- Mon Feb 17, 2020 12:48 pm
- Forum: Balancing Redox Reactions
- Topic: Oxidizing/Reducing Agents
- Replies: 11
- Views: 832
Oxidizing/Reducing Agents
What are oxidizing agents and reducing agents?
- Wed Feb 12, 2020 11:16 am
- Forum: Thermodynamic Definitions (isochoric/isometric, isothermal, isobaric)
- Topic: Reversible vs Irreversible
- Replies: 5
- Views: 434
Re: Reversible vs Irreversible
From what we learned, we only distinguished our calculation for reversible and irreversible to calculate work and the total change in entropy of the universe.
- Tue Feb 11, 2020 11:54 am
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Questions about Equation Sheet
- Replies: 3
- Views: 294
Re: Questions about Equation Sheet
Actually, both of the c values are for monoatomic gases. Cv is used when the reaction is carried out at a constant volume and Cp is used at constant pressure.
[img]Screen%20Shot%202020-02-11%20at%2011.53.40%20AM[/img]
[img]Screen%20Shot%202020-02-11%20at%2011.53.40%20AM[/img]
- Tue Feb 11, 2020 11:44 am
- Forum: Entropy Changes Due to Changes in Volume and Temperature
- Topic: 4F.17
- Replies: 1
- Views: 196
Re: 4F.17
You have to use the deltaS = nCln(T2/T1) equation. First, you use this equation to raise the temperature of water from 85 - 100 (make sure you convert to Kelvin) and use the molar heat capacities at constant pressure of liquid water. Then, you use this equation to lower the temperature of water from...
- Mon Feb 10, 2020 2:21 pm
- Forum: Concepts & Calculations Using Second Law of Thermodynamics
- Topic: Microstates
- Replies: 6
- Views: 266
Re: Microstates
How many microstates does the molecule BF3 have for the B-F bond?
- Mon Feb 10, 2020 2:13 pm
- Forum: Calculating Work of Expansion
- Topic: Reversible and Isobaric
- Replies: 4
- Views: 326
Re: Reversible and Isobaric
The irreversible equation is only used when pressure is constant. If pressure is changing, then you use the reversible equation.
- Wed Feb 05, 2020 12:18 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: 4D.7
- Replies: 1
- Views: 100
Re: 4D.7
Work is being done because there is a change in the moles of gas from reactants to products. When not specified, we assume that the reaction is being done under ideal conditions.
- Tue Feb 04, 2020 8:31 am
- Forum: Entropy Changes Due to Changes in Volume and Temperature
- Topic: R vs C
- Replies: 2
- Views: 123
Re: R vs C
R is the gas constant which is on the equation sheet. C is the specific heat capacity which is usually given in the problem.
- Tue Feb 04, 2020 8:29 am
- Forum: Calculating Work of Expansion
- Topic: when to use...?
- Replies: 4
- Views: 189
Re: when to use...?
You use w=-PV when there is an expansion occurring at a constant external pressure. You use the other equation when there is a change in internal and external pressure for expansion work.
- Mon Feb 03, 2020 10:29 am
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: First Law
- Replies: 5
- Views: 345
Re: First Law
In an isolated system, there is no transfer of heat or matter. In terms of internal energy (U=q+w), there is no heat exchange so q=0 and no change in matter so w=0. This means that the change in internal energy is equal to zero and that it remains constant throughout an experiment on an isolated sys...
- Mon Feb 03, 2020 10:24 am
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: 4C.9A
- Replies: 2
- Views: 97
Re: 4C.9A
The question is essentially asking how much energy it takes to heat the copper kettle which has its own unique specific heat capacity and how much energy it takes to heat the water. You have to perform two calculations to get the total change in energy.
- Wed Jan 29, 2020 9:33 am
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: 4A. 13 Homework
- Replies: 2
- Views: 95
Re: 4A. 13 Homework
Since we are measuring the q of the reaction and we know that heat is being released, the reaction is exothermic, making the q negative.
- Tue Jan 28, 2020 10:02 am
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Bomb calorimetry
- Replies: 3
- Views: 128
Re: Bomb calorimetry
Bomb calorimeters are more sophisticated versions of regular calorimeters. The reaction takes place in a metal vessel with a constant volume. This metal vessel is in water and the temperature change is monitored.
- Tue Jan 28, 2020 9:56 am
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Specific heat as an intensive property
- Replies: 2
- Views: 91
Re: Specific heat as an intensive property
Heat capacity is an extensive property because it varies by the amount of the substance in the situation. Specific heat capacity measures the amount of heat to raise one mole or one gram of a substance by one degree celsius. Since it is per mole or per gram, it can be applied to a variety of situati...
- Mon Jan 27, 2020 9:58 am
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Standard Enthalpy of Diatomic Gases
- Replies: 4
- Views: 212
Standard Enthalpy of Diatomic Gases
Why is the standard enthalpy of diatomic gases (O2) equal to zero?
- Mon Jan 27, 2020 9:48 am
- Forum: Phase Changes & Related Calculations
- Topic: Steam vs water
- Replies: 5
- Views: 204
Re: Steam vs water
How does the delta H of water when it is melting compare to the delta H of water when it is vaporizing?
- Wed Jan 22, 2020 9:23 am
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Le Chatelier's in relation to stability
- Replies: 3
- Views: 138
Re: Le Chatelier's in relation to stability
You can however determine the relative stability of the reactants and products by looking at the Ka value. If it is very large, the products are more stable and if it is very small, the reactants are more stable.
- Tue Jan 21, 2020 11:28 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Checking the approximation of "x"
- Replies: 4
- Views: 158
Re: Checking the approximation of "x"
The approximation is also acceptable if the Ka value is less than 10^-3.
- Tue Jan 21, 2020 11:28 am
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: exothermic reactions
- Replies: 19
- Views: 2047
Re: exothermic reactions
Does this mean that cooling an exothermic reaction will favor the products?
- Mon Jan 20, 2020 9:25 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Pressure goes to less moles of gas explaination
- Replies: 4
- Views: 208
Re: Pressure goes to less moles of gas explaination
When you increase pressure, you are decreasing the volume. This relationship is seen in the PV=nRT equation. A decrease in volume means an increase in concentration. To balance out the increase in concentration, the equilibrium will go toward the side with fewer moles.
- Wed Jan 15, 2020 9:31 am
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Real reason explaining Le Chatelier's principle
- Replies: 4
- Views: 152
Re: Real reason explaining Le Chatelier's principle
When pressure increases, there is a decrease in volume. This inverse relationship can be seen through the PV=nRT equation. When the volume decreases, this means that the concentration (n/V) of all products and reactants increases. Due to the increase in concentration, the reaction will proceed towar...
- Tue Jan 14, 2020 9:48 am
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Temperature change
- Replies: 5
- Views: 119
Re: Temperature change
Why do changes in concentration, pressure, and volume have no effect on the K value?
- Tue Jan 14, 2020 9:46 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: pKa
- Replies: 3
- Views: 124
Re: pKa
A lower pKa value means that the acid is stronger. This value can be useful when comparing relative strength of acids between two weak acids.
- Mon Jan 13, 2020 9:53 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Example 5I.3 (page 556 on pdf)
- Replies: 3
- Views: 100
Re: Example 5I.3 (page 556 on pdf)
Does this removal of x in the expression depend on the value of K?
- Mon Jan 13, 2020 9:51 am
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Change in Pressure
- Replies: 6
- Views: 207
Re: Change in Pressure
When the pressure decreases, the volume increases as they are inversely proportional. This means that the concentration also decreases and to bring the system back to equilibrium, the reaction which creates more moles will be favored.
- Wed Jan 08, 2020 9:14 am
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Pressure changes to equilibrium equations
- Replies: 5
- Views: 259
Re: Pressure changes to equilibrium equations
How does the increasing the pressure of a gas like Helium, which is not active in the chemical equation, affect equilibrium?
- Wed Jan 08, 2020 9:11 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: 5H.3
- Replies: 2
- Views: 163
Re: 5H.3
I believe it is referring to this chart.
[img]Screen%20Shot%202020-01-03%20at%207.53.41%20PM[/img]
[img]Screen%20Shot%202020-01-03%20at%207.53.41%20PM[/img]
- Tue Jan 07, 2020 11:35 am
- Forum: Non-Equilibrium Conditions & The Reaction Quotient
- Topic: Using the "ICE" box
- Replies: 8
- Views: 261
Re: Using the "ICE" box
Solids and liquids are not included because there is generally a large amount of it. This generally does not affect the reactants or products which is why it is left out of the ICE table calculations.
- Mon Jan 06, 2020 4:51 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: The Significance of K
- Replies: 5
- Views: 195
The Significance of K
When do you use Kc vs Kp?
- Mon Jan 06, 2020 4:50 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Equilibrium Constant
- Replies: 4
- Views: 132
Re: Equilibrium Constant
K tells you the proportion of products to reactants present in the reaction and whether there are more products or reactants. When a certain reaction is strongly favored, it means that the K value is greater than 1000.
- Wed Dec 04, 2019 9:59 am
- Forum: Properties & Structures of Inorganic & Organic Bases
- Topic: Bronsted vs Arrhenius Base: J.1(c) and (e)
- Replies: 3
- Views: 339
Re: Bronsted vs Arrhenius Base: J.1(c) and (e)
The OH- in both compounds with accept a proton to create water. Since it is accepting protons, these compounds conform to the definition of a Bronsted base.
- Wed Dec 04, 2019 9:57 am
- Forum: Amphoteric Compounds
- Topic: Amphoteric vs Amphiprotic
- Replies: 9
- Views: 583
Re: Amphoteric vs Amphiprotic
What does the term polyprotic mean? Can a molecule be both polyprotic and amphiprotic?
- Tue Dec 03, 2019 9:42 am
- Forum: Conjugate Acids & Bases
- Topic: 6A.1
- Replies: 6
- Views: 799
Re: 6A.1
For a conjugate base, you remove a hydrogen and add a 1- charge.
- Mon Dec 02, 2019 11:31 am
- Forum: Calculating pH or pOH for Strong & Weak Acids & Bases
- Topic: Water as a acid or base
- Replies: 4
- Views: 307
Re: Water as a acid or base
Water can only be labeled as an acid or a base depending on the reaction. In some cases, it may act as an acid and donate a hydrogen, In other cases, it may act as a base and accept protons. Water has a set constant, Kw, which is 1.01 × 10^-14 at 25 °C.
- Mon Dec 02, 2019 11:21 am
- Forum: Calculating pH or pOH for Strong & Weak Acids & Bases
- Topic: Relative Acidity
- Replies: 2
- Views: 169
Re: Relative Acidity
For binary acids, the atom with a greater radius will be more acidic because it can easily give off an H+ ion. For example, HI is a stronger acid than HF. For oxoacids, the molecule with the greatest amount of oxygens is the most acidic. This is due to the fact the it increases electronegativity, ma...
- Wed Nov 27, 2019 10:37 am
- Forum: Amphoteric Compounds
- Topic: Question 6A.11
- Replies: 5
- Views: 450
Re: Question 6A.11
HCO3- and HPO4 2- can both accept and donate protons. In this way, they are considered amphiprotic and can be used as an acid or a base in the reaction. If they are an acid, they lose the hydrogen in the reaction. If they are basic, they gain a hydrogen in the reaction.
- Tue Nov 26, 2019 1:44 pm
- Forum: Naming
- Topic: Naming coordination compound
- Replies: 5
- Views: 375
Re: Naming coordination compound
This coordinate compound is bisethylenediamine dinitro iron (IV) sulfate.
- Tue Nov 26, 2019 1:36 pm
- Forum: Lewis Acids & Bases
- Topic: Lewis vs Bronsted Acids and Bases
- Replies: 3
- Views: 530
Re: Lewis vs Bronsted Acids and Bases
Lewis and Bronsted Acids and Bases are simply different because of how they define and acid and a base. In Bronsted's definition, an acid is a proton donor and a base is a proton acceptor. In the Lewis definition, an acid is an electron pair acceptor and a base is an electron pair donor. The Lewis t...
- Mon Nov 25, 2019 11:35 am
- Forum: Naming
- Topic: Correct naming conventions
- Replies: 5
- Views: 330
Correct naming conventions
When do you add the suffix -ate to the metal name in a coordination compound?
Re: 9C.3d
I believe that the parentheses are present to be able to identify the molecules in the coordination compound. It is just for ease of reading not a special purpose.
- Wed Nov 20, 2019 8:57 am
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Lone Pairs for AX4E
- Replies: 3
- Views: 198
Lone Pairs for AX4E
For a seesaw shaped molecule, how do you know that the lone pair should be on the equatorial plane and not the axial plane?
- Tue Nov 19, 2019 12:23 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Seesaw shape
- Replies: 1
- Views: 167
Re: Seesaw shape
In the seesaw shape, the bond angles will be <90, <120, and <180.


- Tue Nov 19, 2019 9:21 am
- Forum: Hybridization
- Topic: Stability of bonds
- Replies: 3
- Views: 130
Re: Stability of bonds
Sigma bonds are also more flexible than pi bonds because of their orientation to the atom. Pi bonds do not allow rotation and they are usually the first bonds broken down in a chemical reaction.
- Mon Nov 18, 2019 1:14 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: VSEPR, Molecular Geometry, and Molecular Shape
- Replies: 4
- Views: 370
Re: VSEPR, Molecular Geometry, and Molecular Shape
The VSEPR model gives you the electron geometry and molecular shape. The electron geometry of a molecule is the naming convention based on the number of electron regions. Molecules with 2 electron regions are linear, 3 electron regions are trigonal planar, 4 electron regions are tetrahedral, 5 elect...
- Mon Nov 18, 2019 1:02 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: AXE notation
- Replies: 10
- Views: 683
Re: AXE notation
The AXE notation is useful to organize the types of electron regions in a molecule. For example, SF4 has a seesaw shape. This means it has four bonds and 1 lone pair. This can be described as AX4E in the notation.
- Wed Nov 13, 2019 11:39 am
- Forum: Interionic and Intermolecular Forces (Ion-Ion, Ion-Dipole, Dipole-Dipole, Dipole-Induced Dipole, Dispersion/Induced Dipole-Induced Dipole/London Forces, Hydrogen Bonding)
- Topic: intermolecular forces
- Replies: 8
- Views: 587
Re: intermolecular forces
Dipoles occur when there is an unequal sharing of electrons. Each atom in a molecule has a partial charge which is either negative or positive. When one charge is significantly larger than another, or the electronegativity difference is great, a dipole is produced. Lone pairs on the central atom als...
- Wed Nov 13, 2019 9:08 am
- Forum: Interionic and Intermolecular Forces (Ion-Ion, Ion-Dipole, Dipole-Dipole, Dipole-Induced Dipole, Dispersion/Induced Dipole-Induced Dipole/London Forces, Hydrogen Bonding)
- Topic: London Forces and Dipole-Dipole
- Replies: 5
- Views: 365
Re: London Forces and Dipole-Dipole
London Dispersion forces act on all molecules and atoms. This includes nonpolar molecules, monoatomic gases, and in molecules that also exhibit dipole-dipole interactions.
- Tue Nov 12, 2019 9:01 am
- Forum: Dipole Moments
- Topic: 3f.15
- Replies: 3
- Views: 270
Re: 3f.15
According to the problem, the boiling point of AsF3 is 68C and AsF5 is -53. You can first approach this problem by drawing the Lewis structures for both molecules to find out the intermolecular forces that each molecule has. AsF3 has a lewis structure in which a lone pair on the Arsenic atom. This m...
- Tue Nov 12, 2019 8:51 am
- Forum: Dipole Moments
- Topic: 3F.11
- Replies: 3
- Views: 185
Re: 3F.11
Hydrogen bonds form when hydrogen interacts with nitrogen, oxygen, or fluorine. In this problem, only d can create hydrogen bonds because it has nitrogen and oxygen.
- Mon Nov 11, 2019 11:31 pm
- Forum: Interionic and Intermolecular Forces (Ion-Ion, Ion-Dipole, Dipole-Dipole, Dipole-Induced Dipole, Dispersion/Induced Dipole-Induced Dipole/London Forces, Hydrogen Bonding)
- Topic: Induced Dipole/ Induced Dipole
- Replies: 4
- Views: 290
Re: Induced Dipole/ Induced Dipole
London Dispersion forces are known as induced-dipole–induced-dipole interaction. Interactions that are proportional to 1/r6 are commonly regarded as van der Waals interactions. So London Dispersion forces are a type of van der Waals interaction.
- Wed Nov 06, 2019 8:15 am
- Forum: Formal Charge and Oxidation Numbers
- Topic: Formal Charge
- Replies: 2
- Views: 136
Re: Formal Charge
Electronegativity is the electron pulling power of an atom. The atoms that are more electronegative will want the electron more in real life. The negative formal charge indicates an excess of an electron. Since more electronegative atoms will have a greater power to pull this electron, they will gen...
- Tue Nov 05, 2019 9:12 am
- Forum: Lewis Structures
- Topic: 2C.9
- Replies: 1
- Views: 119
Re: 2C.9
Sulfur is one of the exceptions that Dr. Lavelle mentioned in terms of being able to have an expanded octet. It can bond to 6 atoms and have an expanded octet with 12 electrons.
- Tue Nov 05, 2019 9:08 am
- Forum: Bond Lengths & Energies
- Topic: 2D.9
- Replies: 1
- Views: 100
Re: 2D.9
The Question for 2.D.9: Arrange the cations Rb+1, Be+2, and Sr+2 in order of increasing polarizing power. Explain your reasoning. The cations with the smallest radius and largest charge have the greatest polarizing power. Of the three, beryllium has the smallest radius, so it would have the greatest...
- Mon Nov 04, 2019 9:04 am
- Forum: Electronegativity
- Topic: Trend of Electronegativity
- Replies: 22
- Views: 2125
Re: Trend of Electronegativity
Electronegativity is the electron pulling power of an atom. As you move to the right of the periodic table, the electronegativity increases because atoms get closer and closer to having a full octet. As you move up the periodic table, electronegativity also increases because there is less shielding ...
- Mon Nov 04, 2019 9:00 am
- Forum: Polarisability of Anions, The Polarizing Power of Cations
- Topic: Trends
- Replies: 3
- Views: 260
Re: Trends
Cations with a smaller radius and a more positive charge have greater polarizing power. For example, K+ > K in polarizing power. Anions with a larger radius and a more negative charge have greater polarizability because they have more electrons. So I->Br-, in polarizability.
- Wed Oct 30, 2019 7:39 pm
- Forum: Resonance Structures
- Topic: Determining the Pairing for Lewis Structures
- Replies: 2
- Views: 210
Determining the Pairing for Lewis Structures
How do we know when the hydrogen will be attached to the central atom vs other atoms? Like in the case of HClO2, why is the hydrogen attached to the oxygen and not the chlorine? https://img1.daumcdn.net/thumb/R720x0.q80/?scode=mtistory2&fname=http%3A%2F%2Fcfile7.uf.tistory.com%2Fimage%2F999FF54C...
- Wed Oct 30, 2019 7:34 pm
- Forum: Lewis Structures
- Topic: Radicals
- Replies: 2
- Views: 82
Re: Radicals
Radicals are fairly uncommon because they are highly unstable and reactive. They will generally only stay in that form for a short period of time before interacting with other molecules to get a pair.
- Tue Oct 29, 2019 2:03 pm
- Forum: Ionic & Covalent Bonds
- Topic: Octet Rule
- Replies: 8
- Views: 330
Re: Octet Rule
Professor Lavelle said that phosphorus, sulfur, and chlorine can have expanded octets. This is due to the fact that they have empty p orbitals which can accommodate additional electrons.
- Tue Oct 29, 2019 2:00 pm
- Forum: Ionic & Covalent Bonds
- Topic: Homework 2A.21
- Replies: 1
- Views: 166
Re: Homework 2A.21
Ag is one of the exceptions to the build up principle. It would normally have a configuration of [Kr]4d10 5s1 because the atom would be most stable when the d shell is completely full rather than the s shell. When you remove an electron from Ag, it will be removed from the outermost shell which is 5...
- Mon Oct 28, 2019 7:32 pm
- Forum: Lewis Structures
- Topic: Homework problem 2C.3
- Replies: 5
- Views: 160
Re: Homework problem 2C.3
As of right now, I believe that we do not need to memorize the molecular formula for these molecules since we did not cover it in class. On a test, I think it would be given to us and we would just have to draw the lewis structure.
- Mon Oct 28, 2019 7:29 pm
- Forum: Ionic & Covalent Bonds
- Topic: ionic bonding 2A.13
- Replies: 1
- Views: 110
Re: ionic bonding 2A.13
Chlorine has the ground state electron configuration of [Ne]3s1 3p5. An electron is always removed from the outermost subshell present. In this case, the outermost subshell is 3p.
- Wed Oct 23, 2019 9:02 am
- Forum: Wave Functions and s-, p-, d-, f- Orbitals
- Topic: 1D.23
- Replies: 2
- Views: 205
Re: 1D.23
For b and d, you would go off of the ml number. In a 4p atom in which l=1, there are 3 possibly orbitals orientations as stated above. This means that an electron in the 4p orbital could have the following configurations: n=4, l=1, ml = 1; n=4, l=1, ml = 0, and n=4, l=1, ml = -1. When you are given ...
- Tue Oct 22, 2019 8:23 pm
- Forum: Electron Configurations for Multi-Electron Atoms
- Topic: Aufbau principle
- Replies: 2
- Views: 114
Re: Aufbau principle
Aufbau principle is just rules for how electrons fill atomic orbitals. It states that electrons will always fill the lowest energy state first. For example, an electron will be in the 1s orbital before the 2s orbital. It also states that electrons will be added to different orbitals with parallel sp...
- Tue Oct 22, 2019 8:47 am
- Forum: Electron Configurations for Multi-Electron Atoms
- Topic: Electron Configurations for electrons in the D subshell
- Replies: 5
- Views: 317
Re: Electron Configurations for electrons in the D subshell
This filling of the d orbital over the s orbital will also occur for elements such as chromium. Instead of being [Ar]3d44s2, the electron configuration will be [Ar]3d54s1.
- Mon Oct 21, 2019 8:53 am
- Forum: Electron Configurations for Multi-Electron Atoms
- Topic: Exceptions in Electron Configurations
- Replies: 5
- Views: 196
Re: Exceptions in Electron Configurations
Do all the elements in group 6 and 11 have electron configurations similar to copper and chromium?
- Mon Oct 21, 2019 8:48 am
- Forum: Wave Functions and s-, p-, d-, f- Orbitals
- Topic: terminology - "orbitals", "shells", "subshells"
- Replies: 2
- Views: 139
Re: terminology - "orbitals", "shells", "subshells"
The principal quantum number (n) specifies the shell of the electrons in an atom. For example, 4p corresponds to 4 shells of electrons in the atom. The angular momentum number (l) signifies the sub-shells. The magnetic quantum number describes the amount of orbitals in a sub shell. There are 3 orbit...
- Wed Oct 16, 2019 7:50 am
- Forum: Heisenberg Indeterminacy (Uncertainty) Equation
- Topic: Which equation to use for determining uncertainty?
- Replies: 4
- Views: 241
Which equation to use for determining uncertainty?
Hi, In the textbook, a modified equation for the uncertainty principle is used with the variable h bar (see below). What does this variable signify? Which equation should we use: the one from the textbook or the one that is taught in class? https://scitechdaily.com/images/heisenberg-uncertainty-prin...
- Tue Oct 15, 2019 7:58 am
- Forum: DeBroglie Equation
- Topic: de Broglie vs Heisenberg
- Replies: 2
- Views: 187
Re: de Broglie vs Heisenberg
To add on, the Heisenberg equation is specifically used in regards to uncertainty. The questions will usually ask you what the uncertainty in the velocity is or it will give you a range of values for velocity. This is your main indicator to use the Heisenberg equation over the deBroglie equation.
- Mon Oct 14, 2019 8:19 pm
- Forum: DeBroglie Equation
- Topic: DeBroglie
- Replies: 3
- Views: 211
Re: DeBroglie
DeBroglie's proposed that all particles should be considered to have wave like properties. So he devised the equation: wavelength(lambda) = Planck's constant(h)/ momentum(p). In this equation, we are treating light like a wave and finding the wavelength based on the mass and velocity of the particle.
- Mon Oct 14, 2019 8:16 am
- Forum: Einstein Equation
- Topic: Einstein Equation
- Replies: 4
- Views: 252
Re: Einstein Equation
You use the equation E=hv when you are trying to find out the energy of a photon of light. If you know the frequency of this photon, then you can multiply it by Planck's constant to find the total energy of the photon.
- Mon Oct 14, 2019 8:13 am
- Forum: Properties of Light
- Topic: Memorizing Values for Tests
- Replies: 3
- Views: 205
Re: Memorizing Values for Tests
The values of c (speed), Planck's constant, and Rydberg's constant are on the formula sheet, which will be given to you for every exam.
- Wed Oct 09, 2019 2:13 pm
- Forum: Accuracy, Precision, Mole, Other Definitions
- Topic: Light energy
- Replies: 3
- Views: 241
Re: Light energy
I think it will be helpful to know which types of light have the largest wavelengths and the shortest. For example, radio waves have the greatest wavelength while gamma rays of the shortest. It will essentially be beneficial to know the order. https://www.radio2space.com/wp-content/uploads/2013/07/c...
- Wed Oct 09, 2019 9:22 am
- Forum: Empirical & Molecular Formulas
- Topic: Molecular formula
- Replies: 6
- Views: 2921
Re: Molecular formula
To clarify, when you divide by the least number of moles. The answer you are getting is a molar ratio of atoms in the molecule. This is relative to the other atoms in the molecule. We want this ratio to be a whole number because we can only have a whole number of atoms. To find the molecular formula...
- Tue Oct 08, 2019 11:11 am
- Forum: Properties of Light
- Topic: Light intensity
- Replies: 6
- Views: 211
Re: Light intensity
In terms of wave properties, higher intensity means a greater wave amplitude and therefore a greater amount of energy. In the photoelectric experiment, it was found that increasing the intensity of light did not increase the energy of light. So this experiment supported the idea that light did not h...
- Mon Oct 07, 2019 11:08 am
- Forum: SI Units, Unit Conversions
- Topic: Angstrom(Å)
- Replies: 4
- Views: 226
Re: Angstrom(Å)
An angstrom is not a SI unit. It is used and recognized by many scientists but it is not part of the International System. An angstrom represents 10^-10m. This can be converted to other SI units like nanometers or picometers.
- Mon Oct 07, 2019 11:05 am
- Forum: SI Units, Unit Conversions
- Topic: Molar Ratio
- Replies: 4
- Views: 1356
Re: Molar Ratio
To clarify the statement above, a molar ratio refers to BOTH the stoichemetric coefficients in a chemical equation and the moles of atoms in a molecule. For example, the chemical equation C2H4 + 3O2 → 2CO2 + 2H2O. There is a molar ratio of 2 water molecules to 2 carbon dioxide molecules. Within the ...
- Wed Oct 02, 2019 9:28 am
- Forum: Accuracy, Precision, Mole, Other Definitions
- Topic: Tips on how to write a formula out from the name
- Replies: 9
- Views: 698
Re: Tips on how to write a formula out from the name
There are several polyatomic ions which are good to memorize. There is a list of nomenclature in the beginning of the book which can be useful for future problems.
- Tue Oct 01, 2019 11:49 am
- Forum: Balancing Chemical Reactions
- Topic: Tips for Balancing Chemical Equations [ENDORSED]
- Replies: 14
- Views: 858
Re: Tips for Balancing Chemical Reactions [ENDORSED]
I would first write down the number of atoms of each element that is present for the reactant side and the product side. I would then begin to balance my equation and typically leave hydrogen and oxygen for the end. As I balance, I would readjust the initial numbers that were written at the beginnin...
- Mon Sep 30, 2019 7:53 pm
- Forum: Significant Figures
- Topic: How to Write Out Final Answers
- Replies: 5
- Views: 316
Re: How to Write Out Final Answers
Both 9.87 mL and 9.87 x 10^-3 L have the same value. Regardless of how it is written, the amount that would be measured for the actual experiment would be the same. If the question asks you for the volume in a specific measurement then you would have to choose one or the other.
- Sun Sep 29, 2019 2:33 pm
- Forum: Empirical & Molecular Formulas
- Topic: Empirical Formula
- Replies: 6
- Views: 164
Re: Empirical Formula
While the empirical and molecular formulas can be different, there are situations in which the two formulas are the same. This can be determined by dividing the molar mass of the molecular formula by the molar mass of the empirical formula. If the ratio is 1, the empirical and molecular formulas for...

