Search found 108 matches
- Wed Mar 11, 2020 7:37 am
- Forum: Method of Initial Rates (To Determine n and k)
- Topic: k' vs kr
- Replies: 13
- Views: 841
Re: k' vs kr
to clarify, reverse rate means the reciprocal. k'=1/k
- Wed Mar 11, 2020 7:36 am
- Forum: General Rate Laws
- Topic: Stoichiometric coefficients vs order
- Replies: 4
- Views: 410
Re: Stoichiometric coefficients vs order
The overall reaction order will the same as the stoichiometric coefficient of the rate determining step. Otherwise, each mechanism rate will have its respective order from that step's stoichiometric coefficients.
- Wed Mar 11, 2020 7:34 am
- Forum: Arrhenius Equation, Activation Energies, Catalysts
- Topic: Derivation of Arrhenius Equation
- Replies: 6
- Views: 492
Re: Derivation of Arrhenius Equation
When the prompt talks about temperature, activation energy, and/or the rate constant, it is indicating the use of the Arrhenius equation.
- Tue Mar 10, 2020 1:29 pm
- Forum: Reaction Mechanisms, Reaction Profiles
- Topic: Molecularity
- Replies: 4
- Views: 329
Re: Molecularity
It is a way of categorizing the types of rates just like we can say the rate order of a species or overall reaction.
- Mon Mar 09, 2020 12:07 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: predicting solubility
- Replies: 3
- Views: 306
Re: predicting solubility
Yes, that is correct. To clarify, this is using Le Chatelier's method.
- Thu Mar 05, 2020 5:12 pm
- Forum: General Science Questions
- Topic: Reducing Math Errors
- Replies: 7
- Views: 681
Reducing Math Errors
I constantly find myself having to redo problems because of silly calculator mistakes. Any tips on how I can address this?
- Thu Mar 05, 2020 8:20 am
- Forum: Work, Gibbs Free Energy, Cell (Redox) Potentials
- Topic: finding n in G=-nFE
- Replies: 15
- Views: 1049
Re: finding n in G=-nFE
n is the number of electron transferred which can be found when you balance the redox reaction.
- Wed Mar 04, 2020 3:05 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Ecell vs Ecell°
- Replies: 2
- Views: 244
Re: Ecell vs Ecell°
Ecell°means standard conditions (1M, 25°C, 1atm) Ecell is at nonstandard conditions and is used in the Nernst equation.
- Tue Mar 03, 2020 11:21 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Strongly Reducing vs Oxidizing
- Replies: 2
- Views: 196
Re: Strongly Reducing vs Oxidizing
Reducing power (strength of reducing agent): species undergoes oxidation which is more likely if its standard reduction potential is more negative. Oxidizing power (strength of oxidizing): species undergoes education which is more likely if its standard reduction potential is more positive. The term...
- Mon Mar 02, 2020 7:39 am
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: 6N.7 B
- Replies: 1
- Views: 199
Re: 6N.7 B
I think that is a solution manual error. It should be 2.
- Fri Feb 28, 2020 11:09 am
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Is it spontaneous?
- Replies: 4
- Views: 346
Re: Is it spontaneous?
Ecell=0 means it is a concentration cell. Enaught=0 means the cell potential is 0.
K can't be equal to 0 unless its all solids and liquids in the reaction... otherwise [p]/[r] would always be some number greater or less than 1.
K can't be equal to 0 unless its all solids and liquids in the reaction... otherwise [p]/[r] would always be some number greater or less than 1.
- Thu Feb 27, 2020 9:48 am
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Cell Diagrams
- Replies: 3
- Views: 265
Re: Cell Diagrams
The solid metal is the electrode (conductor) the only exception for liquids is mercury which is a liquid when it functions as an electrode.
- Wed Feb 26, 2020 4:17 pm
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: 6N.3 (c)
- Replies: 1
- Views: 212
Re: 6N.3 (c)
Although we learned about Kc and Kp as separate things, K (or Q) can contain both pressure and concentration. Note, the pressure needs to be in atm or bar (since there's only a 1% difference).
- Tue Feb 25, 2020 10:15 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Midterm question 3D
- Replies: 1
- Views: 231
Re: Midterm question 3D
To do this problem you need to compare the pH and pKa... If the pH is lower than the pKa, then the compound will be protonated. If the pH is higher than the pKa, then the compound will be deprotonated. Here, since pH 6 is greater than pKa 4.75, the compound will be deprotonated will gives CH3COO- an...
- Mon Feb 24, 2020 5:23 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Example 6L.2
- Replies: 1
- Views: 152
Re: Example 6L.2
Based off the cell diagram, the chlorine is the cathode part of the galvanic cell so it is in the reduction part of the redox reaction.
- Thu Feb 20, 2020 9:39 am
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Cell Potential Difference
- Replies: 3
- Views: 245
Re: Cell Potential Difference
These values are given. Remember, electrode potential is the electric potential on an electrode component. In a cell, there is an electrode potential for the cathode and an electrode potential for the anode. The difference between the two electrode potentials equals the cell potential.
- Thu Feb 20, 2020 9:35 am
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Anode
- Replies: 5
- Views: 380
Re: Anode
To clarify, the cathode has a positive potential so relative to the cathode, the anode is negative.
- Wed Feb 19, 2020 11:14 am
- Forum: Balancing Redox Reactions
- Topic: What is being reduced/oxidized in this rxn?
- Replies: 4
- Views: 377
Re: What is being reduced/oxidized in this rxn?
Yes, I think there is an error! That would make sense with the solutions for the half-reactions. In any case, Cl2 is both an oxidizing and reducing agent.
- Tue Feb 18, 2020 9:52 am
- Forum: Balancing Redox Reactions
- Topic: Oxidizing and Reducing Agents
- Replies: 3
- Views: 315
Re: Oxidizing and Reducing Agents
A trick to help you remember which agent is oxidizing and which is redoing is "LEO the lion says GER"
Lose Electron Oxidize
Gain Electron Reduce
Lose Electron Oxidize
Gain Electron Reduce
- Mon Feb 17, 2020 1:12 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: G vs G knot
- Replies: 15
- Views: 1749
Re: G vs G knot
This equation also relates G and G
- Thu Feb 13, 2020 10:41 am
- Forum: Student Social/Study Group
- Topic: Midterm
- Replies: 8
- Views: 712
Re: Midterm
The problems with ICE tables and using the quadratic formula to solve for the equilibrium composition of a chemical reaction were also very reflective of the homework.
- Thu Feb 13, 2020 10:38 am
- Forum: Thermodynamic Definitions (isochoric/isometric, isothermal, isobaric)
- Topic: Isothermal reversible of ideal gas
- Replies: 4
- Views: 374
Re: Isothermal reversible of ideal gas
Isothermal reversible just means the temp stayed constant so q=-w and you can use the equation nrlnv2/v1 to calculate entropy of the system.
- Thu Feb 13, 2020 10:36 am
- Forum: Concepts & Calculations Using Second Law of Thermodynamics
- Topic: deltaSrev/T
- Replies: 2
- Views: 231
Re: deltaSrev/T
When there is no change in temperature, for example phase changes.
- Tue Feb 11, 2020 8:49 am
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: ∆G, ∆H, ∆S
- Replies: 6
- Views: 560
Re: ∆G, ∆H, ∆S
Based on where we are in class, this has been the only relationship established. You can also think of how relative the values of ∆H and ∆S make ∆G negative at which temperatures to determine whether a reaction is spontaneous.
- Tue Feb 11, 2020 8:47 am
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: 4E.5
- Replies: 4
- Views: 275
Re: 4E.5
Drawing out the Lewis structures will indicate the types of bonds. Also it may be helpful to memorize some of the organic compounds. C6H6 is benzene and has ring shape with alternating single and double carbon carbon bonds.
- Thu Feb 06, 2020 2:29 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: Question from Wednesday Lecture
- Replies: 3
- Views: 273
Re: Question from Wednesday Lecture
The condition q=-w and delta U=0 comes from the process being isothermal.
- Thu Feb 06, 2020 2:27 pm
- Forum: Third Law of Thermodynamics (For a Unique Ground State (W=1): S -> 0 as T -> 0) and Calculations Using Boltzmann Equation for Entropy
- Topic: boltzmann constant
- Replies: 2
- Views: 119
Re: boltzmann constant
Yes, its on the equation and constants sheet.
- Thu Feb 06, 2020 2:27 pm
- Forum: Third Law of Thermodynamics (For a Unique Ground State (W=1): S -> 0 as T -> 0) and Calculations Using Boltzmann Equation for Entropy
- Topic: degeneracy
- Replies: 2
- Views: 85
Re: degeneracy
Yes, that's correct. The degeneracy formula is # micro-states^#particles and there are 6.026 x 10^23 particles per mole.
- Tue Feb 04, 2020 5:33 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: 4D.7
- Replies: 1
- Views: 61
Re: 4D.7
The first part of the problem is understanding that -P V=-
V=- nRT so the answer should be -2.48kJ
nRT so the answer should be -2.48kJ
The second part requires solving for U which equals q+w
U which equals q+w
q= H=-318kJ and we just solved for w since w=-P
H=-318kJ and we just solved for w since w=-P V
V
Thus, adding q and w gives the final answer -320kJ
The second part requires solving for
q=
Thus, adding q and w gives the final answer -320kJ
- Tue Feb 04, 2020 5:29 pm
- Forum: Calculating Work of Expansion
- Topic: Problem 4.7a
- Replies: 1
- Views: 84
Re: Problem 4.7a
You will need to balance the chemical equation and look at the change in moles of gas from reactants to products:
C6H6(l) + 15/2O2(g) --> 6CO2(g) + 3H2O(l)
 n = 6-7.5= -1.50mol
n = 6-7.5= -1.50mol
C6H6(l) + 15/2O2(g) --> 6CO2(g) + 3H2O(l)
- Fri Jan 31, 2020 12:32 pm
- Forum: Phase Changes & Related Calculations
- Topic: Phase Changes
- Replies: 17
- Views: 739
Re: Phase Changes
Yes, during a phase change, the temperature doesn't change because the energy is channeled into breaking the intermolecular forces of the substance.
- Thu Jan 30, 2020 10:27 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Strong acids and bases as gases
- Replies: 4
- Views: 235
Re: Strong acids and bases as gases
Acids and bases in water are in aqueous form. However, they could react to produce a gas.
- Wed Jan 29, 2020 9:30 am
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: 4A. 13 Homework
- Replies: 2
- Views: 97
Re: 4A. 13 Homework
Temperature rose as a result of the neutralization reaction which means heat was released which is shown by energy (q) being negative.
- Tue Jan 28, 2020 5:13 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Calculating Work
- Replies: 3
- Views: 108
Re: Calculating Work
Yes, there are two different equations
Irreversible: w=-P V
V
Reversible (more work): w=-nRTln(V2/V1)
Irreversible: w=-P
Reversible (more work): w=-nRTln(V2/V1)
- Mon Jan 27, 2020 9:55 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Sig Figs for Celsius
- Replies: 1
- Views: 62
Re: Sig Figs for Celsius
I would use 3 sig figs because that's following the rule of using the least number of sig figs provided in the prompt. Sometimes the textbook gets the number of sig figs wrong.
- Fri Jan 24, 2020 3:42 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Pressure and Enthalpy
- Replies: 5
- Views: 151
Re: Pressure and Enthalpy
Pressure and enthalpy are directly proportional so as pressure increases so does enthalpy, and vice versa.
- Thu Jan 23, 2020 9:14 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: 6B.11
- Replies: 1
- Views: 71
Re: 6B.11
Use M1V1=M2V2 such that (0.18M)(500mL)=(M2)(5mL) the M2 is the concentration of NaOH which is also the [OH-] of the original solution.
- Wed Jan 22, 2020 8:35 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Percent Ionization
- Replies: 4
- Views: 122
Re: Percent Ionization
It's [H3O+]/[HA] x 100 because the percent ionization is measuring how much the acid [HA] gave up protons [H3O+] the [A-] is not involved.
- Tue Jan 21, 2020 9:03 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Second Ionization in Polyprotic Acid Solutions
- Replies: 2
- Views: 75
Re: Second Ionization in Polyprotic Acid Solutions
The exception is that if Ka2 < Ka1/1000 you can treat the chemical equilibrium reaction as a monoprotic acid. This is because Ka2 is so small relative to Ka1 is has little effect on the concentration of H3O+ formed. Remember, this is because Ka represents the dissociation constant which means the ra...
- Mon Jan 20, 2020 3:30 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: pH of salt solutions
- Replies: 2
- Views: 97
Re: pH of salt solutions
Chemical equilibrium problems of weak acids/bases always use an ICE table because these compounds don't fully dissociate and thus we would use the Ka/Kb value respectively to finish solving the problem.
- Sat Jan 18, 2020 9:16 am
- Forum: Acidity & Basicity Constants and The Conjugate Seesaw
- Topic: Strength of an Acid/Base
- Replies: 2
- Views: 250
Re: Strength of an Acid/Base
Ka and Kb represent the dissociation constant of the acid/base reaction. Thus, the high the Ka or Kb the stronger acid/base because it is more like to give/accept an H+
- Thu Jan 16, 2020 10:49 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Factos effecting Equilibrium
- Replies: 6
- Views: 261
Re: Factors effecting Equilibrium
Thee three we've covered in lecture are concentration, pressure due to change in volume, and temperature.
- Wed Jan 15, 2020 8:51 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: 5.39
- Replies: 1
- Views: 105
5.39
The K value I found in Table 5G2 doesn't match the K value in the answer key so I'm having a hard time doing the problem. Can someone please verify if there's an error in the solution manual?
- Tue Jan 14, 2020 5:18 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: 5g.3 b HOMEWORK
- Replies: 2
- Views: 166
Re: 5g.3 b HOMEWORK
On the class website, it says that there is a typo for this problem so in the given balanced chemical equation the coefficient in front of N2 should be 5. This would also change K in the answer.
- Mon Jan 13, 2020 11:18 am
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Which liquids to use
- Replies: 7
- Views: 203
Re: Which liquids to use
To add on, we don't include a pure liquid in an equilibrium calculation because it acts as the solvent in a chemical reaction and its change in concentration is relatively insignificant so in the K expression, the product concentration and reactant concentration would just cancel out.
- Sat Jan 11, 2020 8:14 pm
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: 5.35
- Replies: 4
- Views: 150
Re: 5.35
Set up an ICE table and fill in the info you are given by reading the initial and equilibrium partial pressures of each compound. Then you can work out the change amount. From there, to write the chemical equation, determine if there is a ratio between the amount of change of A and the amount of cha...
- Sat Jan 11, 2020 8:04 pm
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Effect of pressure on Chemical Equilibrium
- Replies: 3
- Views: 191
Re: Effect of pressure on Chemical Equilibrium
K represents the ratio of equilibrium concentrations of products to reactants so when both the concentration of the products and reactants change proportionally (due to change in pressure due to change in volume), K remains the same.
- Wed Jan 08, 2020 10:12 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Solids and Liquids
- Replies: 3
- Views: 108
Re: Solids and Liquids
Yes, the states will be given because we need to know the states of the chemical compounds in a reaction in order to write a K/Q expression.
- Tue Jan 07, 2020 9:36 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: P(k) and P(q)
- Replies: 2
- Views: 140
Re: P(k) and P(q)
To clarify for both Q (initial conditions) and K (equilibrium conditions): when the compounds are gases use P for partial pressure and when the compounds are in aqueous state use the concentration (brackets). Disregard compounds in solid or liquid phase. Don't use partial pressure and concentration ...
- Mon Jan 06, 2020 9:55 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Water as Liquid and Gas
- Replies: 1
- Views: 90
Re: Water as Liquid and Gas
Water as liquid is a solvent: not included in equilibrium constant because we assume there is a significantly large amount of solvent so that the amount starting (reactant) and amount ending (product) are essentially the same. When we put this in the equilibrium constant equation set up, the numbers...
- Wed Dec 04, 2019 4:57 pm
- Forum: Calculating pH or pOH for Strong & Weak Acids & Bases
- Topic: Strong acids and bases
- Replies: 2
- Views: 234
Re: Strong acids and bases
Yes, there's a list. You can google it, but here's the one I use.
- Wed Dec 04, 2019 11:08 am
- Forum: Biological Examples
- Topic: Hemoglobin - shape and function
- Replies: 2
- Views: 213
Re: Hemoglobin - shape and function
Hemoglobin is four myoglobins. It binds to 4 oxygen molecules and transports the O2. Therefore, it is a polydentate ligand. I don't believe it is chelating.
- Wed Dec 04, 2019 11:01 am
- Forum: Polyprotic Acids & Bases
- Topic: How can you tell if an acid/base is polyprotic?
- Replies: 6
- Views: 1031
Re: How can you tell if an acid/base is polyprotic?
You can tell if something is a polyphonic acid if it has more than one H+ cation. For example H2S,H2SO4,H3PO4. Polyphonic bases can accept more than one proton. It can be recognized by compounds with cations of a greater than +1 charge. This is because OH- is -1 so if the cation is more than +1 we n...
- Wed Dec 04, 2019 10:39 am
- Forum: Lewis Acids & Bases
- Topic: Problem J.9
- Replies: 4
- Views: 190
Re: Problem J.9
How would you do it for ammonia and phosphoric acid? We knew the ionic compound created would be ammonium phosphate. Since ammonium is +1 and phosphate is 3- we need three NH4+ to balance out the PO4 3- From there, work backwards to write the reactants side of the chemical reaction. 3NH3(aq) + H3PO...
- Mon Dec 02, 2019 11:13 am
- Forum: Lewis Acids & Bases
- Topic: 6D.11
- Replies: 1
- Views: 111
6D.11
In this exercise, parts e and f what does it mean by Al(H2O)6 3+ (aq) and Cu(H2O)6 2+ (aq)?
What is the significance of writing both H2O and (aq)?
What is the significance of writing both H2O and (aq)?
- Wed Nov 27, 2019 8:32 am
- Forum: Naming
- Topic: OH2 vs H2O Coordination Complex Chemical Formula
- Replies: 3
- Views: 171
OH2 vs H2O Coordination Complex Chemical Formula
I noticed both of these chemical formulas being written for coordination complexes. Is one more correct than the other? How do we know which one to use? An example is 9C3 parts c and d.
- Wed Nov 27, 2019 8:29 am
- Forum: Naming
- Topic: Naming Coordination Compound with Iron
- Replies: 4
- Views: 475
Re: Naming Coordination Compound with Iron
Ferrate is the Latin name. We use it to indicate that the coordination complex ion has an overall negative charge. Some other common ones we discussed in discussion are
copper: cuprate
gold: aurate
lead: plumbate
copper: cuprate
gold: aurate
lead: plumbate
- Tue Nov 26, 2019 4:29 pm
- Forum: Conjugate Acids & Bases
- Topic: Acids and Bases
- Replies: 10
- Views: 571
Re: Acids and Bases
To add on, you can tell if the reaction involves a weak/strong acid/base by checking if there is a single/double equilibrium arrow.
- Mon Nov 25, 2019 8:04 pm
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: Polydentate Ligands
- Replies: 2
- Views: 211
Re: Polydentate Ligands
Yes, "poly" just means more than 1. In lecture we specified the number of bonds using other prefixes (bi/tri/tetra) but polydentate works for all of them.
- Mon Nov 25, 2019 8:00 pm
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: Radicals
- Replies: 3
- Views: 217
Re: Radicals
I'm not sure if we need to know it, but to my knowledge you just count the radical as another thing around the central atom so it increases the coordination number by 1.
- Wed Nov 20, 2019 9:06 pm
- Forum: Ionic & Covalent Bonds
- Topic: CO2 and H20
- Replies: 4
- Views: 452
Re: CO2 and H20
Jorge Ramirez_4H wrote:Why is co2 non polar? isn't based on electronegativity?
CO2 is non polar because the dipoles cancel out. The difference in electronegativity between C and O are in equal magnitude and opposite direction.
- Wed Nov 20, 2019 9:04 pm
- Forum: Interionic and Intermolecular Forces (Ion-Ion, Ion-Dipole, Dipole-Dipole, Dipole-Induced Dipole, Dispersion/Induced Dipole-Induced Dipole/London Forces, Hydrogen Bonding)
- Topic: IMF strength
- Replies: 4
- Views: 278
Re: IMF strength
Ion-dipole is stronger that hydrogen bonding because it has an ion. Think of hydrogen bonding as a special (and stronger) type of dipole-dipole intermolecular force.
- Wed Nov 20, 2019 9:03 pm
- Forum: Polarisability of Anions, The Polarizing Power of Cations
- Topic: Melting Point
- Replies: 3
- Views: 344
Re: Melting Point
higher polarizing power --> greater dipole-dipole forces --> stronger intermolecular forces --> higher melting point
- Wed Nov 20, 2019 9:01 pm
- Forum: Sigma & Pi Bonds
- Topic: Resonance Structures
- Replies: 2
- Views: 226
Re: Resonance Structures
Technically, every compound could have a resonance structure then. When we draw Lewis structures, we pick the most realistic/stable structure such that there are few formal charges. In the case of SO2, there would be 2 sigma and 2 pi bonds because sulfur forms two double bonds, one with each oxygen.
- Mon Nov 18, 2019 9:17 pm
- Forum: Dipole Moments
- Topic: non polar dipole moments
- Replies: 2
- Views: 198
Re: non polar dipole moments
Dipole-dipole interactions occur anywhere there is a covalent bond and difference in electronegativity (basically anytime two different elements form a covalent bond). A dipole moment is when there is a NET dipole-dipole interaction. This occurs if the dipole-dipole interactions don't cancel out. In...
- Mon Nov 18, 2019 9:11 pm
- Forum: Lewis Structures
- Topic: lone pairs
- Replies: 7
- Views: 733
Re: lone pairs
Also, check the octet rule to see if the central atom can hold more than 8 e- (whether those be lone pairs and/or bonds).
- Sun Nov 17, 2019 3:53 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Lone pairs and bond angles
- Replies: 4
- Views: 246
Re: Lone pairs and bond angles
Yes, the repulsion of a lone pair pushes the atoms bonded to the central atom closer together. This is because the electron cloud of a lone pair can spread over a larger volume than a bonding pair can, because a bonding pair (or several bonding pairs in a multiple bond) is held in place by two atoms...
- Sat Nov 16, 2019 8:00 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Tool to Memorize VSEPR
- Replies: 3
- Views: 117
Tool to Memorize VSEPR
I found this video very useful! https://www.youtube.com/watch?v=GnXSGR-UEDI
- Thu Nov 14, 2019 11:32 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: 2E1 - Molecules with bent geometry
- Replies: 7
- Views: 422
Re: 2E1 - Molecules with bent geometry
For this problem, I found it useful to look at the bond angles. The first example, bent, has angles 120 degrees and we know that for a bent electron arrangement, there must be 2 bonding pairs and 1 or 2 lone pairs. Therefore, in the diagram, when only 2 bonding pairs are shown, we know we must have ...
- Thu Nov 14, 2019 11:26 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Lewis Structures & VSEPR
- Replies: 9
- Views: 495
Re: Lewis Structures & VSEPR
Drawing the Lewis structure can help you count up the total lone pairs and the total bonding pairs on a central atom which then correlates to the molecular shape and electron arrangement of a molecule. Remember, however, that you must be able to draw the most correct Lewis structure for this method ...
- Wed Nov 13, 2019 8:08 am
- Forum: Interionic and Intermolecular Forces (Ion-Ion, Ion-Dipole, Dipole-Dipole, Dipole-Induced Dipole, Dispersion/Induced Dipole-Induced Dipole/London Forces, Hydrogen Bonding)
- Topic: Intermolecular Forces vs. Chemical Bonds
- Replies: 3
- Views: 258
Intermolecular Forces vs. Chemical Bonds
How are intermolecular forces different from chemical bonds?
- Tue Nov 12, 2019 8:44 am
- Forum: Dipole Moments
- Topic: 3f.1
- Replies: 3
- Views: 214
Re: 3f.1
A good tip to have in mind is carbon generally only has London dispersion intermolecular forces. This is due to the symmetry it creates from the bonds it forms with other elements (carbon is tetravalent). However, in addition to that, there could be other intermolecular forces among the other elemen...
- Tue Nov 12, 2019 8:39 am
- Forum: Coordinate Covalent Bonds
- Topic: Determining bonds
- Replies: 4
- Views: 486
Re: Determining bonds
A quick tip to always keep in mind is hydrogen is a nonmetal so when you see HCl/HBr/HI it's tempting to say it's an ionic bond because H has a +1 charge and halogens have a -1 charge and they're on opposite sides of the periodic table like most ionic compounds. However, that'd be incorrect, these c...
- Wed Nov 06, 2019 5:21 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Midterm
- Replies: 1
- Views: 115
Re: Midterm
The info sheet online says pen!
- Wed Nov 06, 2019 2:17 pm
- Forum: Lewis Structures
- Topic: Lewis Structure
- Replies: 4
- Views: 340
Re: Lewis Structure
1. Tally up total of valence electrons in compound. 2. Choose which element goes in the middle. It's usually the one that shows up the least in the chemical formula and/or it is the least electronegative. 3. Draw single bonds from central atom to the atoms you have remaining. Add lone pairs to satis...
- Tue Nov 05, 2019 10:57 am
- Forum: Ionic & Covalent Bonds
- Topic: molecular polarity
- Replies: 3
- Views: 226
molecular polarity
Why is CO more polar than BO? This was on a workshop review worksheet.
- Mon Nov 04, 2019 8:53 pm
- Forum: Bohr Frequency Condition, H-Atom , Atomic Spectroscopy
- Topic: Rydberg Dino Nuggets problem 11
- Replies: 1
- Views: 147
Re: Rydberg Dino Nuggets problem 11
A) The equations you need to use is E=-hR/n^2 and deltaE=Efinal-Einitial then you can relate E to frequency using the equation E=hv.
B) deltaE will be negative. To fall energy levels, energy is being emitted. Energy of the photon, however, will be positive (it can never be negative).
B) deltaE will be negative. To fall energy levels, energy is being emitted. Energy of the photon, however, will be positive (it can never be negative).
- Mon Nov 04, 2019 8:58 am
- Forum: Electron Configurations for Multi-Electron Atoms
- Topic: aufbau principle
- Replies: 4
- Views: 275
Re: aufbau principle
Micah3J wrote:Will we need to know the f block for the midterm?
No, we need to know s, p, and d.
- Sat Nov 02, 2019 8:32 am
- Forum: Wave Functions and s-, p-, d-, f- Orbitals
- Topic: Energy of orbitals
- Replies: 2
- Views: 85
Energy of orbitals
In lecture and discussion, we've been saying one orbital has higher/lower energy than another orbital. I understand we can tell by looking at n, the principal quantum number. However, what does the energy of an orbital actually mean?
- Wed Oct 30, 2019 3:14 pm
- Forum: Ionic & Covalent Bonds
- Topic: Likely Charge for Ions to Form
- Replies: 3
- Views: 277
Re: Likely Charge for Ions to Form
An easy trick to remember is to count the groups across the period table, disregarding the transition metals, the order is +1,+2,+3 the 4th group is "neutral" then -3,-2,-1, noble gases don't form ions.
- Wed Oct 30, 2019 3:12 pm
- Forum: Ionic & Covalent Bonds
- Topic: Format of midterm?
- Replies: 12
- Views: 682
Re: Format of midterm?
Here is what's posted online:
Midterm is 2 hours, 8 questions.
Midterm covers all material up to the end of Focus 2D in Outline 3:
Chemical Bonds.
Questions will come from the Homework and Online Assessments.
Midterm is 2 hours, 8 questions.
Midterm covers all material up to the end of Focus 2D in Outline 3:
Chemical Bonds.
Questions will come from the Homework and Online Assessments.
- Wed Oct 30, 2019 3:10 pm
- Forum: Octet Exceptions
- Topic: Octet confusion
- Replies: 2
- Views: 94
Re: Octet confusion
Yes, I believe what you stated is corrected. Basically, my TA explained that starting with elements in the 3rd row, though they don't have electrons on the d orbital, they have access to the 3d subshell which is how the additional electrons after the 8th are getting stored there.
- Mon Oct 28, 2019 9:39 pm
- Forum: Electron Configurations for Multi-Electron Atoms
- Topic: Electron Configuration of Tungsten
- Replies: 2
- Views: 716
Electron Configuration of Tungsten
Why is the electron configuration of tungsten [Xe] 4f14 5d4 6s2 instead of [Xe] 4f14 5d5 6s1? I thought we were supposed to follow the rule about d5 and d10 so that there was at least one electron in each d orbital to make the atom more stable?
- Mon Oct 28, 2019 8:42 am
- Forum: Administrative Questions and Class Announcements
- Topic: Midterm
- Replies: 2
- Views: 110
Midterm
What is the format of the midterm exam? (How many questions?/is it multiple choice/free response?) Thanks!
- Fri Oct 25, 2019 8:50 am
- Forum: Ionic & Covalent Bonds
- Topic: Octet Rule Exceptions
- Replies: 3
- Views: 180
Octet Rule Exceptions
Why are H, He, Li, and Be exceptions for the octet rule?
- Fri Oct 25, 2019 8:47 am
- Forum: Heisenberg Indeterminacy (Uncertainty) Equation
- Topic: Question 12 Heisenberg Uncertainty Principle Online Module
- Replies: 1
- Views: 149
Question 12 Heisenberg Uncertainty Principle Online Module
Please help me answer the question in the photo attached. Thanks!
- Wed Oct 23, 2019 12:17 pm
- Forum: Electron Configurations for Multi-Electron Atoms
- Topic: Writing Electron Configurations Help
- Replies: 3
- Views: 175
Re: Writing Electron Configurations Help
The textbook answer is in increasing n (principal quantum number) for bismuth [Xe] 4f14 5d10 6s2 6p3. Which is the convention in my experience. Dr. Lavelle might have been explaining how to write electron configurations in increasing energy to understand which orbitals get filled first with electron...
- Tue Oct 22, 2019 11:13 am
- Forum: Trends in The Periodic Table
- Topic: Ionization Energy Across a Period
- Replies: 3
- Views: 135
Re: Ionization Energy Across a Period
As we go across a period, the atomic radius decreases so there is a greater attraction between the negatively charged electrons and positively-charged nucleus thus it will take more energy to remove a valence electron (greater ionization energy).
- Mon Oct 21, 2019 3:04 pm
- Forum: Electron Configurations for Multi-Electron Atoms
- Topic: orbitals
- Replies: 5
- Views: 169
Re: orbitals
We will not need to draw the orbitals. Planes refers to the axes the orbitals can be oriented. This shows how for s we can only have 1 (one orientation for a spherical shape) so one orbital in 2 subshell. While p we have three (xy/xz/yz) so there are 3 orbitals in the p subshell. And the patterns co...
- Sat Oct 19, 2019 12:00 pm
- Forum: Electron Configurations for Multi-Electron Atoms
- Topic: HW 1D.23
- Replies: 2
- Views: 162
HW 1D.23
How many orbitals can have the following quantum numbers in an atom: (a)n=2,l=1;(b)n=4,l=2,ml=-2; (c)n=2;(d)n=3,l=2,ml=-1?
- Sat Oct 19, 2019 11:58 am
- Forum: Electron Configurations for Multi-Electron Atoms
- Topic: HW 1D.19
- Replies: 2
- Views: 99
HW 1D.19
How many orbitals are present in the (a) 4p-subshell; (b) 3d-subshell; (c) 1s-subshell; (d) 4f-subshell of an atom?
- Fri Oct 18, 2019 12:11 pm
- Forum: Wave Functions and s-, p-, d-, f- Orbitals
- Topic: wave functions/orbitals/quantum numbers
- Replies: 2
- Views: 149
wave functions/orbitals/quantum numbers
How are wave functions/orbitals connected to quantum numbers? I'm confused... isn't the wave function about the whole atom and the quantum number refers to individual electrons?
- Fri Oct 18, 2019 10:41 am
- Forum: Heisenberg Indeterminacy (Uncertainty) Equation
- Topic: What does the equation actually show?
- Replies: 3
- Views: 175
Re: What does the equation actually show?
We won't really be using the Heisenberg Indeterminacy Equation to do calculations. It is rather to explain that electron(s) can't be located inside the nucleus of an atom, there is a physical limit to the minimum size that atoms can exist, and furthermore, that electrons must have wavelike propertie...
- Wed Oct 16, 2019 12:13 pm
- Forum: Properties of Light
- Topic: 1B #9
- Replies: 5
- Views: 170
Re: 1B #9
Find energy per photon using the equation E=hc/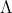
Multiply by 64 because there are 32 Watts per second and the prompt asks for 2 seconds (1 W = 1 J/s)
You will get 1.4 x 10^20 photons, then convert to moles using Avogadro's number.
The final answer will be 2.3 x 10^-4 mol photons
Multiply by 64 because there are 32 Watts per second and the prompt asks for 2 seconds (1 W = 1 J/s)
You will get 1.4 x 10^20 photons, then convert to moles using Avogadro's number.
The final answer will be 2.3 x 10^-4 mol photons
- Wed Oct 16, 2019 12:08 pm
- Forum: Properties of Light
- Topic: 1B #19
- Replies: 2
- Views: 161
Re: 1B #19
The de Broglie wavelength equation relates h to p and p is equal to mass times velocity. Therefore you can substitute p (momentum) for the mass of the proton/neutron and the velocity is the same for either particle (as given in the prompt). h is Planck's constant.
- Mon Oct 14, 2019 6:37 pm
- Forum: *Shrodinger Equation
- Topic: psi vs psi^2
- Replies: 7
- Views: 421
Re: psi vs psi^2
As McKenna mentioned, the concept isn't a big thing to stress over understanding. The main point of today's lecture is for us to know that orbitals aren't just shapes drawn when talking about electrons or stating what the electron is s/p/d/etc. as we might have in high school. They are actually math...
- Fri Oct 11, 2019 10:28 am
- Forum: Bohr Frequency Condition, H-Atom , Atomic Spectroscopy
- Topic: Help on A1.15
- Replies: 4
- Views: 286
Re: Help on A1.15
In the textbook there is a small paragraph that says in the UV region of the spectrum, n1 is equal to 1. From there you can solve for n2.
- Fri Oct 11, 2019 10:25 am
- Forum: Quantum Numbers and The H-Atom
- Topic: Rydberg Equation
- Replies: 2
- Views: 83
Re: Rydberg Equation
The Rydberg equation is sometimes hard for us to tell which level should be n1 and which should be n2. The method we learned in lecture is based off our understanding of the conservation of energy so that the change in energy is always E final - E initial and E=-hR/n^2 and we can tell if the change ...
- Wed Oct 09, 2019 9:11 am
- Forum: Properties of Electrons
- Topic: electron energy
- Replies: 4
- Views: 199
Re: electron energy
The energy needed to remove an e- is known as the ionization energy. It varies depending on the properties of the atom, or in this case the properties of the metal surface. Typically we are given components of the problem that we can use to solve for the unknown. For example, we are given the freque...
- Wed Oct 09, 2019 9:00 am
- Forum: Photoelectric Effect
- Topic: Work Function
- Replies: 3
- Views: 285
Re: Work Function
The work function from what we've been introduced to in lecture shows the minimum energy required to eject an electron from a metal surface. It also shows a relationship between the energy of the incoming photon and the kinetic energy of the e- since it is the difference between the energy of the in...

