Search found 102 matches
- Sun Mar 15, 2020 11:47 pm
- Forum: Reaction Mechanisms, Reaction Profiles
- Topic: Reverse reaction rate?
- Replies: 4
- Views: 485
Re: Reverse reaction rate?
Look at the rate constants of the forward and reverse reactions. The equilibrium constant K is equivalent to the forward rate constant divided by the reverse rate constant.
- Sun Mar 15, 2020 11:44 pm
- Forum: Entropy Changes Due to Changes in Volume and Temperature
- Topic: Difference in volume and temperature
- Replies: 6
- Views: 596
Re: Difference in volume and temperature
You use them in the appropriate situations, so if a system does expansion work at constant temperature, then you probably have to use the volume change equation. If the system changes temperature at constant volume, it's likely you have to use the temperature change equation.
- Sun Mar 15, 2020 11:40 pm
- Forum: Calculating Standard Reaction Entropies (e.g. , Using Standard Molar Entropies)
- Topic: enthalpy constants?
- Replies: 6
- Views: 586
Re: enthalpy constants?
I believe they pertained to kinetic energy.
- Sun Mar 15, 2020 11:40 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Electrode size [ENDORSED]
- Replies: 2
- Views: 323
Re: Electrode size [ENDORSED]
As long as the electrode is still in the solution and has not completely degraded, it shouldn't affect voltage of a galvanic cell.
- Sun Mar 15, 2020 11:39 pm
- Forum: Kinetics vs. Thermodynamics Controlling a Reaction
- Topic: Activation Energy and Energy of a Reaction
- Replies: 10
- Views: 635
Re: Activation Energy and Energy of a Reaction
It's important to consider whether the reaction is exothermic or endothermic when trying to determine the outcome of changing temperature on a reaction. If the reaction is is exothermic, raising temperature will favor the reverse reaction, which means it will increase the rate of the reverse reactio...
- Sun Mar 08, 2020 11:43 pm
- Forum: Reaction Mechanisms, Reaction Profiles
- Topic: K’
- Replies: 4
- Views: 378
Re: K’
k' is the rate constant for the reverse of a reaction. It can be used in conjunction with k to calculate the equilibrium constant by setting the two rates equal to each other.
- Sun Mar 08, 2020 11:42 pm
- Forum: Zero Order Reactions
- Topic: Graphs
- Replies: 13
- Views: 1400
Re: Graphs
I think the most important thing pertaining to graphs is that you understand the relationship between the variables. For example, the graph of a 0 order reaction is a linear relationship between reactant concentration and time, whereas the graph of a 1st order reaction is a linear relationship betwe...
- Sun Mar 08, 2020 11:35 pm
- Forum: Arrhenius Equation, Activation Energies, Catalysts
- Topic: purpose
- Replies: 4
- Views: 371
Re: purpose
The Arrhenius equation relates the rate constant to temperature and activation energy.
- Sun Mar 08, 2020 11:33 pm
- Forum: Work, Gibbs Free Energy, Cell (Redox) Potentials
- Topic: non ideal gases
- Replies: 6
- Views: 664
Re: non ideal gases
When finding n though, make sure you don't accidentally double the value. You only need to count the electrons on one side of the redox reaction, as they are being transferred. So if 2 electrons are being transferred from Fe to Cl for example, make sure you don't count 4 from the 2 being transferred...
- Sun Mar 08, 2020 11:29 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Cell Diagram
- Replies: 6
- Views: 470
Re: Cell Diagram
You include liquids if they are involved in the reactions in the voltaic cell. However, if one electrode has a chemical in aqueous solution, but water is not part of any of the reactions in the voltaic cell, you need not write water into the cell diagram. However, the chemical in aqueous solution sh...
- Fri Feb 28, 2020 1:28 am
- Forum: Entropy Changes Due to Changes in Volume and Temperature
- Topic: Test 2
- Replies: 2
- Views: 326
Test 2
My TA told me that test 2 covers only material after the midterm, so that the thermochemistry on it would only be delta G stuff. Is this true, or should we still go over entropy material?
- Fri Feb 28, 2020 1:27 am
- Forum: Balancing Redox Reactions
- Topic: Redox reactions with single reactant
- Replies: 2
- Views: 184
Redox reactions with single reactant
In the book, there are several problems in which a species acts as both an oxidizing agent and a reducing agent. Are we going to be responsible for knowing this for the test? I don't remember going over this in lecture.
- Fri Feb 28, 2020 1:21 am
- Forum: Balancing Redox Reactions
- Topic: How to tell if its being reduced or oxidized
- Replies: 15
- Views: 2208
Re: How to tell if its being reduced or oxidized
When looking at redox reactions or electrochemical cells, I find it helpful to label each element with its own charge, as it clears up any confusion that may arise, as you stated. If you label Mn with +7 in MnO 4 - , it becomes clear that it is being reduced, as it goes from a +7 charge to a +2 char...
- Fri Feb 28, 2020 1:19 am
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: concentration cells
- Replies: 5
- Views: 387
Re: concentration cells
Concentration cells are galvanic cells in which both the anode and cathode have the same element.
- Fri Feb 28, 2020 1:18 am
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Anode vs Cathode
- Replies: 15
- Views: 884
Re: Anode vs Cathode
You can also examine the reactions happening at the electrodes. The oxidation reaction happens at the anode and the reduction reaction happens at the cathode. If it is still unclear, you can also check reduction potential, in the sense that E has to be positive for the galvanic cell to work spontane...
- Sun Feb 23, 2020 11:47 pm
- Forum: Balancing Redox Reactions
- Topic: half reaction
- Replies: 10
- Views: 706
Re: half reaction
In acidic solution, you add water to balance out the oxygen in the half reaction.
In basic solution, you do the same as above but you also add OH- to the side with H+ in order to neutralize the acidity of that side, which makes water.
In basic solution, you do the same as above but you also add OH- to the side with H+ in order to neutralize the acidity of that side, which makes water.
- Sun Feb 23, 2020 11:44 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: w max
- Replies: 3
- Views: 390
Re: w max
I'm not entirely sure, but how I understand it is that with constant temperature and pressure, the energy change is equivalent to the work done, assuming no heat is released. This would be w(max), or the maximum amount of work possible.
- Sun Feb 23, 2020 11:41 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Salt Bridges
- Replies: 3
- Views: 293
Re: Salt Bridges
The salt bridge allows for positive charges to be equalized in the anode half cell and negative charges to be equalized in the cathode half cell. This prevention of charge buildup allows the galvanic cell to continue working.
- Sun Feb 23, 2020 11:38 pm
- Forum: Balancing Redox Reactions
- Topic: basic solution
- Replies: 3
- Views: 281
Re: basic solution
This is because if you use the normal method, you are saying there is a bunch of H+ in a basic solution, which makes no sense. By changing the H+ to H 2 O by combining it with OH - and adding OH - to the other side, the reaction equation now states that the reaction occurs with a bunch of OH - in ba...
- Sun Feb 23, 2020 11:36 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: galvanic cell function
- Replies: 2
- Views: 156
Re: galvanic cell function
The work is done by the flow of electrons. So if there were some electronic gadget on the connection between the cathode and anode, it would be powered by the cell.
- Tue Feb 18, 2020 2:40 am
- Forum: Administrative Questions and Class Announcements
- Topic: Is this course curved?
- Replies: 7
- Views: 570
Re: Is this course curved?
From what I can gather, the midterm was quite difficult for most people, so there's a chance a few points may be added to normalize the grades of the class.
- Tue Feb 18, 2020 2:39 am
- Forum: Balancing Redox Reactions
- Topic: Odd number of electrons
- Replies: 3
- Views: 357
Re: Odd number of electrons
It shouldn't happen, I don't think. In redox reactions we tend to focus on individual atoms being reduced or oxidized, so it shouldn't share electrons.
- Tue Feb 18, 2020 2:37 am
- Forum: Administrative Questions and Class Announcements
- Topic: Midterm Nerves
- Replies: 7
- Views: 480
Re: Midterm Nerves
Something I found helpful on the amphetamines problem was to list what you know. It helped me get insight on how to approach the problem, even when the solution wasn't obvious.
- Tue Feb 18, 2020 2:36 am
- Forum: Administrative Questions and Class Announcements
- Topic: Test 2
- Replies: 11
- Views: 772
Re: Test 2
It won't be cumulative. I believe it picks up from Gibbs free energy.
- Tue Feb 18, 2020 2:35 am
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: delta G vs. delta G naught
- Replies: 6
- Views: 457
Re: delta G vs. delta G naught
delta G naught is under standard conditions. if conditions are not standard, use delta G.
- Sun Feb 09, 2020 12:55 am
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Homework 4A7
- Replies: 2
- Views: 138
Re: Homework 4A7
When the question says "its", it is referring to the entire system, which includes the kettle and the water.
- Sun Feb 09, 2020 12:51 am
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Confused about Heat of Combustion
- Replies: 2
- Views: 112
Re: Confused about Heat of Combustion
I don't think you need to memorize this. This is just saying the heat of combustion for x moles of ethane = moles of ethane * (heat of combustion/1 mole of ethane).
- Sun Feb 09, 2020 12:48 am
- Forum: Entropy Changes Due to Changes in Volume and Temperature
- Topic: Irreversible and Reversible Processes
- Replies: 2
- Views: 114
Re: Irreversible and Reversible Processes
In an irreversible process, heat is transferred out of the system, which increases the entropy of the universe. However, in reversible processes, theoretically, no heat is lost, which means the entropy of the universe does not change.
- Sun Feb 09, 2020 12:45 am
- Forum: Phase Changes & Related Calculations
- Topic: 4C.13
- Replies: 1
- Views: 194
Re: 4C.13
The transfer of heat only stops when both objects are at the same temperature. This means the ice will continue to gain heat until it is melted and at the same temp as the water, or the water has frozen over and is the same temp as the ice. Since I'm assuming the water won't freeze, energy will be c...
- Sun Feb 09, 2020 12:43 am
- Forum: Third Law of Thermodynamics (For a Unique Ground State (W=1): S -> 0 as T -> 0) and Calculations Using Boltzmann Equation for Entropy
- Topic: Entropy vs. moles
- Replies: 3
- Views: 636
Re: Entropy vs. moles
Yes, if you add matter to a system, the entropy will increase. More moles means more molecules which means more microstates for the molecules to be in.
- Wed Jan 29, 2020 10:32 am
- Forum: Non-Equilibrium Conditions & The Reaction Quotient
- Topic: approximation
- Replies: 4
- Views: 210
Re: approximation
I assume you're referring to calculations using equilibrium constants. If the equilibrium constant you are using is less than 10 -3 , you can usually assume that subtracting x does not change a constant very much. However, this does not mean that x is zero. For example, if given 10 -5 = x 2 /(0.15-x...
- Wed Jan 29, 2020 10:28 am
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Moles
- Replies: 8
- Views: 567
Re: Moles
Make sure you only count gaseous moles though, as liquids and solids are not noticeably affected by pressure changes to the equilibrium.
- Wed Jan 29, 2020 10:01 am
- Forum: Thermodynamic Systems (Open, Closed, Isolated)
- Topic: Calorimeters as Isolated Systems
- Replies: 2
- Views: 169
Re: Calorimeters as Isolated Systems
In a bomb calorimeter, the item being burned is considered part of the system. An isolated system cannot exchange matter and energy with its surroundings, but it can exchange matter and energy within itself.
- Wed Jan 29, 2020 9:55 am
- Forum: Phase Changes & Related Calculations
- Topic: Steam burn and ice burns
- Replies: 1
- Views: 130
Re: Steam burn and ice burns
The heat from the burned skin would be transferred to the ice cube by conduction, and the ice cube would begin to melt. However, if it were applied after the skin was already burned, there would already be tissue damage, and the ice cube wouldn't reverse the burn.
- Wed Jan 29, 2020 9:50 am
- Forum: Phase Changes & Related Calculations
- Topic: Phase change and temp
- Replies: 8
- Views: 312
Re: Phase change and temp
It is important to distinguish between breaking bonds and breaking the intermolecular attractions between molecules in a substance. During phase changes, the chemical bonds in the molecules of the substance aren't actually being broken. Rather, the energy added is being used to break the attractions...
- Sat Jan 25, 2020 1:38 am
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Calculation methods
- Replies: 6
- Views: 297
Re: Calculation methods
How is method 4 different than method 3?
- Sat Jan 25, 2020 1:36 am
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: exothermic reactions
- Replies: 19
- Views: 2121
Re: exothermic reactions
Using Le'Chatlier's principle, the system will work to counteract the changes brought upon it. Therefore, heating the reaction favors the reactants, as this uses up the heat. Conversely, cooling the reaction favors the products, as it would release heat, restoring the initial state.
- Sat Jan 25, 2020 1:34 am
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: k<10^-3
- Replies: 9
- Views: 377
Re: k<10^-3
Something to remember is that strong acids don't have K values. In the k expression, the denominator would be essentailly 0, which is an invalid expression.
- Sat Jan 25, 2020 1:31 am
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: When to use Standard enthalpies of formation
- Replies: 3
- Views: 124
Re: When to use Standard enthalpies of formation
To account for non-standard conditions, you just add the enthalpy changes required to get the reactants into their respective states in the conditions given.
- Sat Jan 25, 2020 1:26 am
- Forum: Phase Changes & Related Calculations
- Topic: Bond Enthalpies
- Replies: 5
- Views: 293
Re: Bond Enthalpies
The second way Dr. Lavelle suggested to measure the enthalpy of a reaction was to use bond enthalpies. Essentially, you would find how much energy is required to break every bond in the reactants and add how much energy is released when every bond in the products is formed. However, a shortcut is to...
- Tue Jan 14, 2020 11:00 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Kp vs Kc
- Replies: 4
- Views: 131
Re: Kp vs Kc
It is important to remember that because of the Ideal Gas Law, gases in equilibrium have concentrations proportional to their partial pressures. This is why both may be used in equilibrium constant calculations, although not with each other.
- Tue Jan 14, 2020 10:58 pm
- Forum: Ideal Gases
- Topic: Law of Mass Action
- Replies: 3
- Views: 191
Re: Law of Mass Action
In short, the Law of Mass Action just states that for a set temperature, the equilibrium constant K does not change.
- Tue Jan 14, 2020 10:57 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Equilibrium Constant
- Replies: 4
- Views: 136
Re: Equilibrium Constant
What exactly do you mean by aqueous solution? From what I understand, we include ions in solution in equilibrium constants. However, pure liquids and solids are excluded because they have an activity of 1, and equilibrium constants are technically calculated with activity, not concentration.
- Tue Jan 14, 2020 10:54 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: When to approximate
- Replies: 5
- Views: 161
Re: When to approximate
I'm not sure if this rule applies in this class, but my TA gave out a worksheet today in which we were supposed to approximate that subtracting X from a concentration doesn't change the concentration. The worksheet asks for you to check if the concentration of the product, or X, was less than 5% of ...
- Tue Jan 14, 2020 10:51 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: ICE Chart
- Replies: 5
- Views: 352
Re: ICE Chart
If you see reverse and forwards reactions as arbitrary, I think it makes ICE tables easier. There is not a universal way to tell which way of a reaction is forward, it just depends on how its written. If ICE tables make sense to you for forwards reactions, just imagine the reverse reaction as the fo...
- Fri Jan 10, 2020 1:20 am
- Forum: Ideal Gases
- Topic: Adding reactions
- Replies: 4
- Views: 269
Re: Adding reactions
When adding chemical equations, the reactants stay reactants and the products stay products. Therefore, in the combined equation, all reactants would be in the denominator and would be multiplied together. A similar logic can be applied to the products for the numerator. This is equivalent to multip...
- Fri Jan 10, 2020 1:16 am
- Forum: Ideal Gases
- Topic: PV=nRT equation manipulation
- Replies: 13
- Views: 730
Re: PV=nRT equation manipulation
If there were, would that be a problem? Assuming the gas constant R was given in the correct units and the temperature was specified, I would think there would be enough information to solve a problem even if both partial pressures and concentrations were part of the information provided.
- Fri Jan 10, 2020 1:14 am
- Forum: Ideal Gases
- Topic: 5G.11
- Replies: 6
- Views: 229
Re: 5G.11
Yes, the reaction quotient Q can indicate the direction of the reaction by comparing it to K. If Q < K, then the products side is favored and the forward reaction will proceed, and vice versa if Q > K.
- Wed Jan 08, 2020 9:19 am
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Pressure changes to equilibrium equations
- Replies: 5
- Views: 264
Re: Pressure changes to equilibrium equations
I think that increasing the partial pressure of a gas that doesn't participate in a reaction such as He has a similar effect as increasing the pressure of the system, assuming the volume of the reaction vessel is kept constant. If the partial pressure of He is increased without any of the other part...
- Wed Jan 08, 2020 9:13 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: AV Mods Chem Equilibrium Part 1B Post Exam [ENDORSED]
- Replies: 2
- Views: 254
- Tue Jan 07, 2020 12:18 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: AV Mods Chem Equilibrium Part 1B Post Exam [ENDORSED]
- Replies: 2
- Views: 254
AV Mods Chem Equilibrium Part 1B Post Exam [ENDORSED]
In the post exam, there is a question 19b, but no question 19a. However, the question references the previous part, and doesn't provide sufficient information to solve it in its current form. I also tried using figures from question 18, but the answer I got does not match any of the available choice...
- Thu Dec 05, 2019 8:41 pm
- Forum: Conjugate Acids & Bases
- Topic: Double Arrows
- Replies: 3
- Views: 353
Re: Double Arrows
It is always used in equilibriums to show a dynamic constant state of simultaneous reaction.
- Thu Dec 05, 2019 2:08 pm
- Forum: Interionic and Intermolecular Forces (Ion-Ion, Ion-Dipole, Dipole-Dipole, Dipole-Induced Dipole, Dispersion/Induced Dipole-Induced Dipole/London Forces, Hydrogen Bonding)
- Topic: CH4 versus CCl4 (Boiling Point)
- Replies: 3
- Views: 6108
Re: CH4 versus CCl4 (Boiling Point)
CH 4 has weaker London forces than CCl 4 because it is smaller and has fewer electrons, which means the intermolecular forces in CH 4 are weaker than those in CCl 4 since both molecules are nonpolar. This means it takes less energy to break the attractions between molecules in a sample of CH 4 , res...
- Thu Dec 05, 2019 2:04 pm
- Forum: Acidity & Basicity Constants and The Conjugate Seesaw
- Topic: using Ka and Pka
- Replies: 4
- Views: 342
Re: using Ka and Pka
i think for 14A, you only need to know how to calculate those values. In 14B, you can use those values in calculations and potentially get other values such as pH from Ka and pKa, but for now you don't need to worry about that.
- Thu Dec 05, 2019 2:02 pm
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: chelate
- Replies: 3
- Views: 215
Re: chelate
As I understand it,if a complex has the central metal atom as a part of a ring, it is a chelate.
- Thu Dec 05, 2019 1:45 pm
- Forum: Calculating pH or pOH for Strong & Weak Acids & Bases
- Topic: Acid/Base Trends
- Replies: 6
- Views: 420
Re: Acid/Base Trends
Why is HF a weaker acid than HI? When an acid dissociates, the bond must be broken between the H + and the conjugate base. If the bond is strong, which it is in the case of HF, this makes it harder and less of the acid dissociates, resulting in less H + present in the resulting solution and a highe...
- Fri Nov 29, 2019 3:13 am
- Forum: Conjugate Acids & Bases
- Topic: Acids and Bases
- Replies: 10
- Views: 577
Re: Acids and Bases
More generally, equilibrium arrows are used whenever there is an equilibrium present. This can be in reactions involving acids and bases, or just any reaction in general that can have an equilibrium such as the equilibrium between NO2 and N2O4.
- Fri Nov 29, 2019 3:11 am
- Forum: Acidity & Basicity Constants and The Conjugate Seesaw
- Topic: calculating pKa and pKb
- Replies: 2
- Views: 247
Re: calculating pKa and pKb
The conjugate acid is [C5H5NH]+.
- Fri Nov 29, 2019 3:07 am
- Forum: Properties & Structures of Inorganic & Organic Acids
- Topic: Acid Strength
- Replies: 6
- Views: 432
Re: Acid Strength
It's worth noting that while H2SO4 is a strong acid, once it gives off its first proton, it becomes a weak acid. The HSO4- ion acts as a weak acid, as while it can give off its second proton, it usually doesn't.
- Fri Nov 29, 2019 3:04 am
- Forum: Calculating pH or pOH for Strong & Weak Acids & Bases
- Topic: Strength and Weakness
- Replies: 12
- Views: 1130
Re: Strength and Weakness
I feel like this is obvious, but it hasn't been mentioned so I'll say it. You can also compare pH (lower pH means stronger acid).
- Fri Nov 29, 2019 3:03 am
- Forum: Lewis Acids & Bases
- Topic: HCl vs HF
- Replies: 19
- Views: 1415
Re: HCl vs HF
Bond strengths is the most-correct reason that HCl is a stronger acid compared to HF. If we only consider electronegativity, we only focus on how strongly F can pull H's electron, which doesn't necessarily imply how easy it is to pull the proton off of HF.
- Fri Nov 22, 2019 2:23 am
- Forum: Determining Molecular Shape (VSEPR)
- Topic: AXE Format
- Replies: 34
- Views: 1283
Re: AXE Format
I don't think it matters, as if you don't, common sense tells you that the subscript is 1, and if you do, you definitely wouldn't get marked down for it.
- Fri Nov 22, 2019 2:22 am
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Test 2
- Replies: 14
- Views: 792
Re: Test 2
knowing bond angles of vsepr structures and how lone pairs can affect those bond angles is probably a good idea
- Fri Nov 22, 2019 2:19 am
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Shape of Diatomic Molecules
- Replies: 5
- Views: 464
Re: Shape of Diatomic Molecules
do diatomic molecules have a bond angle?
- Fri Nov 22, 2019 2:18 am
- Forum: Interionic and Intermolecular Forces (Ion-Ion, Ion-Dipole, Dipole-Dipole, Dipole-Induced Dipole, Dispersion/Induced Dipole-Induced Dipole/London Forces, Hydrogen Bonding)
- Topic: Hydrogen Bonding Atoms
- Replies: 6
- Views: 336
Re: Hydrogen Bonding Atoms
it can bond with any N, O, or F atom with lone pairs available.
- Fri Nov 22, 2019 2:15 am
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: Transplatin
- Replies: 3
- Views: 296
Re: Transplatin
The fact that transplatin can't bind to DNA means that it doesn't stop replication, which is the purpose of chemotherapy drugs.
- Thu Nov 14, 2019 9:30 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: why are double bonds equally weighted as single ones when drawing models?
- Replies: 10
- Views: 1987
Re: why are double bonds equally weighted as single ones when drawing models?
No. It's important to remember that in molecules with both single and multiple bonds, there only exist hybrid bonds of equal energy, and the localized double bonds depicted in lewis structures only exist as a limitation of lewis structures. This is why resonance structures exist: because the multipl...
- Thu Nov 14, 2019 9:27 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Drawing Molecular Structures
- Replies: 8
- Views: 555
Re: Drawing Molecular Structures
It's important to remember that the shaded/dashed triangles still represent bonds, just they help show a 3D shape in 2D.
- Thu Nov 14, 2019 9:22 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Molecular Shape Name
- Replies: 17
- Views: 904
Re: Molecular Shape Name
Some shapes come from others, so it might not be necessary to explicitly memorize every shape. For example, bent comes from a tetrahedral shape with 2 lone pairs, but you don't have have to necessarily memorize bent. Instead, just think about a tetrahedral shape and remove 2 atoms.
- Thu Nov 14, 2019 9:10 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: VSEPR Formula
- Replies: 2
- Views: 215
Re: VSEPR Formula
Generally, an A represents the central atom, an X represents an atom bonded to it, and an e represents a lone pair. For example, the formula of a tetrahedral shape would be AX4, and the formula for a bent shape would be AX2e2.
- Thu Nov 14, 2019 9:06 pm
- Forum: Dipole Moments
- Topic: Dipole Moment to figure out polarity
- Replies: 3
- Views: 297
Re: Dipole Moment to figure out polarity
A good way to think about this is to compare a known polar molecule to a known nonpolar molecule. Take H 2 O and CH 4 for example. We know water is polar, and we can see that the net dipole moments do not cancel, and the molecule is left with a positive dipole by the hydrogen atoms and a negative di...
- Fri Nov 08, 2019 2:20 am
- Forum: Lewis Structures
- Topic: Drawing Lewis Structures
- Replies: 18
- Views: 711
Re: Drawing Lewis Structures
I learned that you should try to minimize the absolute charge on the atoms in the molecule/ion. Therefore, if an ion has an overall +1 charge, it should be achieved by 0 charges and a single +1 charge, instead of say a -3 and a +4 charge.
- Fri Nov 08, 2019 2:18 am
- Forum: Lewis Structures
- Topic: Formal Charges
- Replies: 15
- Views: 1010
Re: Formal Charges
Recall the rules for drawing Lewis structures. The central atom is the one that is the least electronegative, which means it has the least electron pulling power. Therefore, it would probably be best for it to not have a negative charge.
- Fri Nov 08, 2019 2:17 am
- Forum: Octet Exceptions
- Topic: General principles of octet exception
- Replies: 7
- Views: 352
Re: General principles of octet exception
The expanded octet comes from access to d orbitals, which can be used to create additional bonds to the 4 allowed by the s and p orbitals. In general, if an atom in a molecule can achieve a lower formal charge and make the molecule have a more logical formal charge by making more than 4 bonds (there...
- Fri Nov 08, 2019 2:15 am
- Forum: Octet Exceptions
- Topic: Lewis Acids and Bases?
- Replies: 11
- Views: 561
Re: Lewis Acids and Bases?
A friend gave me the mnemonic "Lewis has e" which reminds me that Lewis acids and bases focus on electron exchange. After this, I just think about H+, which is acidic, and how it can accept electrons. Therefore, Lewis acids are electron acceptors.
- Fri Nov 08, 2019 2:13 am
- Forum: Administrative Questions and Class Announcements
- Topic: Midterm --> Final Concepts
- Replies: 3
- Views: 246
Re: Midterm --> Final Concepts
I believe the final is cumulative, but it is generally a good idea to remember the concepts covered so far, as they may come up in future chem classes.
- Fri Nov 01, 2019 3:04 am
- Forum: Lewis Structures
- Topic: Best way to go about drawing Lewis structures
- Replies: 7
- Views: 353
Re: Best way to go about drawing Lewis structures
I suppose the trial and error gets easier with experience, as it is possible to identify patterns such as certain functional groups or complex ions in a structure.
- Fri Nov 01, 2019 2:58 am
- Forum: Electronegativity
- Topic: electronegative
- Replies: 6
- Views: 461
Re: electronegative
If one atom is more electronegative than the other, the electrons will spend more time on average around that atom, and the character of the bond would become more ionic.
- Fri Nov 01, 2019 2:57 am
- Forum: Resonance Structures
- Topic: hybrid structure
- Replies: 4
- Views: 176
Re: hybrid structure
Be wary of resonance structures any time you see a multiple bond, as you just have to think about if you could put the multiple bond somewhere else.
- Fri Nov 01, 2019 2:54 am
- Forum: Octet Exceptions
- Topic: Exceptions to the Octet Rule Question
- Replies: 3
- Views: 174
Re: Exceptions to the Octet Rule Question
They do not always violate the octet rule. A simple counterexample would be HCl, which follows the rule.
- Fri Nov 01, 2019 2:51 am
- Forum: Ionic & Covalent Bonds
- Topic: Electronegativity
- Replies: 7
- Views: 284
Re: Electronegativity
A trick I use is to just memorize that fluorine is the most electronegative element, and note its position on the periodic table.
- Fri Oct 25, 2019 1:05 am
- Forum: Bond Lengths & Energies
- Topic: Bond lengths
- Replies: 15
- Views: 1051
Re: Bond lengths
Debora Fernandez Clemente_ 4H wrote:would the bond length vary when it is a double or triple bond?
Yes! Multiple bonds will be shorter than single bonds.
- Fri Oct 25, 2019 1:03 am
- Forum: Ionic & Covalent Bonds
- Topic: Electron Affinity
- Replies: 5
- Views: 313
Re: Electron Affinity
While my high school AP Chem teacher emphasized that this is a very unscientific way to think about it, I think it makes sense to think about electron affinity as how badly a neutral atom "wants" an electron. So for elements such as fluorine, which is so close to achieving a stable noble g...
- Fri Oct 25, 2019 12:59 am
- Forum: Trends in The Periodic Table
- Topic: Homework Question 1F.19
- Replies: 5
- Views: 299
Re: Homework Question 1F.19
Does anyone else find the wording a little ambiguous? Because I think you can make the argument that fluorine is extremely reactive too, due to how close it is to a noble gas electron configuration, but fluorine is definitely not an s-block metal. In this case, fluorine readily takes electrons from ...
- Fri Oct 25, 2019 12:46 am
- Forum: Ionic & Covalent Bonds
- Topic: Resonance
- Replies: 12
- Views: 495
Re: Resonance
Another way to look at this (I think) is to look at bond order. If a molecule has resonance, its bond order will be higher than a molecule with only single bonds. In other words, its "average" bond strength will be in between that of a single bond and a double bond, and since multiple bond...
- Mon Oct 21, 2019 1:47 pm
- Forum: Properties of Electrons
- Topic: Problem 1E.5
- Replies: 3
- Views: 258
Problem 1E.5
I'm having a little trouble with problem 1E.5. It reads as follows: Which of the following statements are true for many-electron atoms? If false, explain why. (a) The effective nuclear charge Z eff is independent of the number of electrons present in an atom. (b) Electrons in an s-orbital are more e...
- Fri Oct 18, 2019 1:41 am
- Forum: Einstein Equation
- Topic: Mass of a proton and neutron
- Replies: 3
- Views: 198
Re: Mass of a proton and neutron
I believe there's a table on Lavelle's course website with those masses.
- Fri Oct 18, 2019 1:38 am
- Forum: Properties of Light
- Topic: 1A9 Table
- Replies: 2
- Views: 167
Re: 1A9 Table
Reading involves visible light.
Getting a dental x-ray involves x-rays.
Using a microwave results in microwave radiation.
Tanning involves UV rays.
Getting a dental x-ray involves x-rays.
Using a microwave results in microwave radiation.
Tanning involves UV rays.
- Fri Oct 18, 2019 1:34 am
- Forum: Einstein Equation
- Topic: Joules units
- Replies: 6
- Views: 955
Re: Joules units
In the formula E = hv, E is measured in Joules and v is measured in Hz, or s^-1. By dimensional analysis, we see that by multiplying kg x m^2 / s by 1/s, we get a units of kg x m^2/s^2. Since Joules is a measure of energy/work, it is equivalent to force x distance. Breaking this down further, force ...
- Fri Oct 18, 2019 1:25 am
- Forum: Photoelectric Effect
- Topic: Problem 1B.3
- Replies: 4
- Views: 167
Re: Problem 1B.3
Part of the experiment demonstrating the photoelectric effect revealed that more electrons can be ejected from metal by shining light on it if the wavelength of the light was increased. If light was viewed as a wave, we would expect increasing intensity to correlate to more electrons being ejected. ...
- Fri Oct 18, 2019 1:22 am
- Forum: Properties of Light
- Topic: Unit for Wavelength
- Replies: 34
- Views: 2529
Re: Unit for Wavelength
Wavelength refers to the length of a wave, which is measured in meters. Just in case you needed the conversions, 1 nm = 10^-9 m. I also think that these conversions will be provided on tests.
- Fri Oct 11, 2019 1:41 am
- Forum: Limiting Reactant Calculations
- Topic: Theoretical yield
- Replies: 5
- Views: 410
Re: Theoretical yield
Dimensional analysis will reveal that the units cancel out, and you end up with an answer in grams when you multiply the 2 quantities.
- Fri Oct 11, 2019 1:40 am
- Forum: Quantum Numbers and The H-Atom
- Topic: Atomic Spectra Question
- Replies: 3
- Views: 214
Re: Atomic Spectra Question
I thought it was by wavelength, not frequency, although I guess because they're proportional it doesn't matter.
- Fri Oct 11, 2019 1:36 am
- Forum: Properties of Light
- Topic: Emission/line spectrum
- Replies: 3
- Views: 188
Re: Emission/line spectrum
I think in the future, we may be asked what color a specific wavelength of light given off is (i.e if an electron emits a photon with a wavelength of 400, what color is the light?)
- Fri Oct 11, 2019 1:34 am
- Forum: Heisenberg Indeterminacy (Uncertainty) Equation
- Topic: Uncertainty in Speed [ENDORSED]
- Replies: 31
- Views: 17781
Re: Uncertainty in Speed [ENDORSED]
One additional thing for my reply, make sure you convert nm to m.
- Fri Oct 11, 2019 1:33 am
- Forum: Heisenberg Indeterminacy (Uncertainty) Equation
- Topic: Uncertainty in Speed [ENDORSED]
- Replies: 31
- Views: 17781
Re: Uncertainty in Speed [ENDORSED]
I think it goes like this:
( p)(
p)( x) = (1/2)(h/2
x) = (1/2)(h/2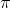 )
)
( p) = m(
p) = m( v)
v)
From here you can plug in (0.01)*(0.05 nm) for x and solve.
x and solve.
(
(
From here you can plug in (0.01)*(0.05 nm) for
- Sat Oct 05, 2019 12:52 pm
- Forum: Properties of Light
- Topic: Electrical Field
- Replies: 1
- Views: 94
Electrical Field
Can someone explain 1.3 to me? The question is as follows: Which of the following happens when the frequency of electromagnetic radiation decreases? Explain your reasoning. (a) The speed of the radiation decreases. (b) The wavelength of the radiation decreases. (c) The extent of the change in the el...
- Wed Oct 02, 2019 12:10 am
- Forum: Empirical & Molecular Formulas
- Topic: Clarification on the Vitamin C Example
- Replies: 6
- Views: 265
Re: Clarification on the Vitamin C Example
My best guess would be that rounding caused the masses of the individual elements to be a little smaller than they really are, which creates a small discrepancy in the summed mass and the total mass. In real life, our instruments are not always perfect, and this might be trying to show that.
- Wed Oct 02, 2019 12:06 am
- Forum: Significant Figures
- Topic: General Rules to Help with Sig Figs
- Replies: 18
- Views: 1067
Re: General Rules to Help with Sig Figs
I really think 5293 has 4 sig figs, not 5. I think if it was 5293.0, it would have 5 sig figs.
- Tue Oct 01, 2019 10:06 pm
- Forum: Limiting Reactant Calculations
- Topic: 2 Limiting Reactants
- Replies: 9
- Views: 383
Re: 2 Limiting Reactants
Suppose the activation energy is too high for the reaction to proceed without a catalyst. Does that make a difference?

