Search found 100 matches
- Sat Mar 14, 2020 5:54 pm
- Forum: General Rate Laws
- Topic: Stoichiometric Coefficients
- Replies: 3
- Views: 278
Re: Stoichiometric Coefficients
If that step is determined to be the slowest step (rate determining step), then yes the molecularity would define the rate law.
- Sat Mar 14, 2020 5:25 pm
- Forum: General Science Questions
- Topic: Final Overview
- Replies: 5
- Views: 498
Re: Final Overview
There will probably be a lot on kinetics, since that is one of the last topics we learned and have not yet been tested on.
- Sat Mar 14, 2020 5:21 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Adding water [ENDORSED]
- Replies: 4
- Views: 386
Re: Adding water [ENDORSED]
Adding pure water to the cathode or to the anode would decrease the concentration at the cathode/anode, thus increasing the cell potential because theres a bigger difference between the concentration of the anode/cathode (only if you only dilute one, not if you dilute both the same amount).
- Sat Mar 14, 2020 5:17 pm
- Forum: General Rate Laws
- Topic: half life
- Replies: 5
- Views: 453
Re: half life
For those types of problems, you just have to use the integrated rate law equations (specified for which order reaction it is). In that case, your [A] will be (1/16)[A]initial, or whatever fraction of the initial the problem specifies.
- Mon Mar 09, 2020 12:28 pm
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: Concentration Cells
- Replies: 3
- Views: 326
Concentration Cells
Are there any specific equations that we can use to apply to problem the have concentration cells? (ie: test question?) What's the best method to go about solving problems with them?
- Mon Mar 09, 2020 12:13 pm
- Forum: Reaction Mechanisms, Reaction Profiles
- Topic: Steady-State Approach
- Replies: 4
- Views: 315
Steady-State Approach
What exactly about the steady state approach will we have to know for the final?
- Mon Mar 09, 2020 12:06 pm
- Forum: Ideal Gases
- Topic: Partial Pressure
- Replies: 13
- Views: 749
Re: Partial Pressure
On the left side of the equation (reactant side) we have 3 moles of reactants and on the right side of the equation (product side) we have two moles of products. Decreasing the volume of a system increases the pressure of the system, and thus the reaction wants to shift to the side with fewer moles ...
- Mon Mar 09, 2020 12:03 pm
- Forum: Non-Equilibrium Conditions & The Reaction Quotient
- Topic: cubic equations, assumption
- Replies: 3
- Views: 307
Re: cubic equations, assumption
Usually if we are given a situation in which we can't ignore the x concentration (cannot follow the assumption), we will be able to solve the equation for x using the quadratic formula. I don't think Dr. Lavelle would give us a cubic equation which we would have to solve without the assumption.
- Fri Mar 06, 2020 12:38 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: inert gases
- Replies: 6
- Views: 429
Re: inert gases
If it is one of the substances being oxidized or reduced, then you would include it on its respective side (anode/left side if it's being oxidized or cathode/right side if it's being reduced).
- Fri Mar 06, 2020 12:27 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Inert electrode
- Replies: 9
- Views: 598
Inert electrode
When do you add an inert electrode (such as platinum) in the cell notation?
- Sun Mar 01, 2020 4:52 pm
- Forum: Balancing Redox Reactions
- Topic: moles of electrons transferred
- Replies: 4
- Views: 386
Re: moles of electrons transferred
When given a balanced chemical reaction, you look at the oxidation numbers of the elements you believe to be involved in redox, and from there obtain the half reactions. Electrons are lost in oxidation and gained in reduction. Once you figure out which element has lost or gained electrons, you can s...
- Sun Mar 01, 2020 4:31 pm
- Forum: First Order Reactions
- Topic: First Order Reaction Rate
- Replies: 4
- Views: 297
Re: First Order Reaction Rate
Since in a first order reaction, the coefficient of the reactants is one, and "a" represents the coefficient of the reactant, thus "a" = 1. Thus, the equation becomes -d[A]/dt = k[A]. Normally on the right side of the equation [A] would be raised to the "n" power, but s...
- Sun Mar 01, 2020 4:13 pm
- Forum: Work, Gibbs Free Energy, Cell (Redox) Potentials
- Topic: delta G = -nFE(cell) Variables
- Replies: 2
- Views: 1745
Re: delta G = -nFE(cell) Variables
Delta G is the standard change in Gibb's free energy, n is the number of electrons involved in the redox reaction (from the balanced equation), F is Faraday's constant (can be found on equation sheet), and E is the standard cell potential (measured in volts).
- Sun Mar 01, 2020 4:07 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Galvanic v Electrolytic Cell
- Replies: 2
- Views: 209
Re: Galvanic v Electrolytic Cell
The cell diagrams and the location of the cathode, anode, are the same in both an electrolytic cell and a galvanic cell. For both, oxidation still happens at the anode and reduction still happens at the cathode. In a galvanic cell, the anode has a negative charge and the cathode has a positive charg...
- Sun Mar 01, 2020 3:55 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Cathode and Anode of Electrolytic Cells
- Replies: 4
- Views: 292
Re: Cathode and Anode of Electrolytic Cells
The cell diagrams and the location of the cathode, anode, are the same in both an electrolytic cell and a galvanic cell. For both, oxidation still happens at the anode and reduction still happens at the cathode. In a galvanic cell, the anode has a negative charge and the cathode has a positive charg...
- Thu Feb 20, 2020 9:25 am
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: 5.55 (b)
- Replies: 1
- Views: 142
Re: 5.55 (b)
I believe you have to calculate for the limiting reactant because you don't actually have the equilibrium value of H2O. Once you find out that graphite is the limiting reactant, you can calculate the concentration of H2O consumed and thus have a value of H2O to use (when making the ice table, otherw...
- Thu Feb 20, 2020 9:20 am
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Anode
- Replies: 5
- Views: 371
Re: Anode
The electrons flow from the anode to the cathode. Therefore, the cathode must have a positive charge to attract the electrons, and thus the anode must have a negative charge so that the electrons are not attracted to it.
- Thu Feb 20, 2020 9:15 am
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Cathode to the Right Rule
- Replies: 6
- Views: 476
Re: Cathode to the Right Rule
Yes, usually for galvanic cells the anode is on the left and the cathode is on the right. As mentioned above, at the anode oxidation takes place and at the cathode reduction takes place.
- Thu Feb 20, 2020 9:14 am
- Forum: Thermodynamic Definitions (isochoric/isometric, isothermal, isobaric)
- Topic: Standard Enthalpy of Formation
- Replies: 3
- Views: 354
Re: Standard Enthalpy of Formation
Usually a question asking to calculate the change in enthalpy of a reaction will give you a table of the enthalpies of formation, which would require you to use that method to find the change in enthalpy. Sometimes it is easier to use the enthalpies of formation to calculate enthalpy change because ...
- Thu Feb 20, 2020 9:12 am
- Forum: Balancing Redox Reactions
- Topic: oxidation number
- Replies: 3
- Views: 339
Re: oxidation number
In a way it is the charge, but it more specifically is the number of electrons lost or gained by an atom. For example, in KCl, there is no charge on the overall molecule, but K has an oxidation number of +1 because it is donating one electron and Cl has an oxidation number of -1 because it is accept...
- Tue Feb 18, 2020 11:48 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: relationship between K and temp
- Replies: 2
- Views: 226
Re: relationship between K and temp
The relationship between K and temperature also depends on whether or not the reaction is endothermic or exothermic. If a reaction is endothermic and you are increasing the temperature, it is as if you are increasing the reactants, and thus the equilibrium reaction will favor the products (forward r...
- Tue Feb 18, 2020 11:44 am
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Homework question 5G.13
- Replies: 2
- Views: 219
Re: Homework question 5G.13
A reaction can only be spontaneous if its delta g is negative, as that is the definition of a spontaneous reaction, so you would be correct.
- Tue Feb 18, 2020 11:37 am
- Forum: Balancing Redox Reactions
- Topic: Free Electrons?
- Replies: 3
- Views: 195
Re: Free Electrons?
The half reactions are part of the larger overall redox reaction, which consists of the half reactions of oxidation and reduction. Thus, you are only look at half of the overall equation when you're looking at a half-reaction. That singular electron gained by Ag in a reduction was lost by oxidation ...
- Tue Feb 18, 2020 11:35 am
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: deltaG in relation to K
- Replies: 3
- Views: 187
Re: deltaG in relation to K
In addition to what other people have replied, when K = 1 (which is very rare), delta g equals 0.
- Tue Feb 18, 2020 11:28 am
- Forum: Administrative Questions and Class Announcements
- Topic: Midterm Nerves
- Replies: 7
- Views: 476
Re: Midterm Nerves
Honestly, the method that has helped me the most when I am faced with problems I am unsure of how to start (aka many of those on the midterm) is writing down every equation related to what you think the question is asking. Thus, if the question mentions or wants you to find entropy, write down every...
- Tue Feb 11, 2020 11:58 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: Balanced Chemical Equations
- Replies: 4
- Views: 437
Re: Balanced Chemical Equations
I'm not exactly sure why, but usually for formation reactions you usually want it to be where you only form one mole of the compound in question.
- Tue Feb 11, 2020 11:57 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: q=n*delta H
- Replies: 5
- Views: 567
Re: q=n*delta H
Both q=m c delta T and q = m delta h can be calculated using moles - it just depends on what units either c or delta h are given in. On the equation sheet given by Professor Lavelle, delta H is given in molar units, so you have to convert your mass to moles. Usually c for q = m c delta t is given in...
- Tue Feb 11, 2020 11:53 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Hess' Law with formulas of combustion and formation
- Replies: 1
- Views: 91
Hess' Law with formulas of combustion and formation
When writing equations for combustion and formation (and when we need to use their enthalpies/entropies to calculate the entropy or enthalpy of the overall reaction), should I always have the equation forming one mole of the compound in question (other molar coefficients in fractions)?
- Tue Feb 11, 2020 11:49 pm
- Forum: Concepts & Calculations Using Second Law of Thermodynamics
- Topic: Delta S
- Replies: 6
- Views: 364
Delta S
Why is delta S equal to zero in a reversible reaction?
- Tue Feb 11, 2020 7:03 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: Delta U = 0
- Replies: 8
- Views: 552
Delta U = 0
What are the specific cases when delta U = 0?
- Wed Jan 29, 2020 2:28 pm
- Forum: Calculating Work of Expansion
- Topic: Negative sign on work equation
- Replies: 3
- Views: 113
Negative sign on work equation
Why is there a negative on the w = - P times delta V? Is there any situation where this would not be the case?
- Wed Jan 29, 2020 2:24 pm
- Forum: Calculating Work of Expansion
- Topic: When to use the different work equations?
- Replies: 1
- Views: 46
When to use the different work equations?
When do we use the equation work = -P times the change in volume versus the integration formula?
- Wed Jan 29, 2020 2:22 pm
- Forum: Calculating Work of Expansion
- Topic: How does compression/expansion change the energy in a system?
- Replies: 2
- Views: 82
Re: How does compression/expansion change the energy in a system?
When you compress the system (with a piston), you're doing work on the system. If you let the piston move out, the system is doing work on the surroundings, and the system loses energy as it is the system that is expending that energy to do the work.
- Wed Jan 29, 2020 12:14 pm
- Forum: Ideal Gases
- Topic: Temperature
- Replies: 17
- Views: 788
Re: Temperature
Usually the question will specify. If you are not sure whether to use Kelvin or Celsius, an easy way of checking is to see what the units are for the other numbers or constants in your equation to see if they have Kelvin or Celsius in them. Depending on which one they use, you may or may not have to...
- Wed Jan 29, 2020 12:09 pm
- Forum: Identifying Acidic & Basic Salts
- Topic: acidic and basic salts
- Replies: 3
- Views: 250
Re: acidic and basic salts
When a salt dissociates, the cation and anion can form a weak acid/base when it reacts with water. For example, when NaCl dissociates, it forms Na+ and Cl-. Na+ reacts with H2O to form NaOH, a strong base. Since it is a strong base, NaOH will fully dissociate in water again to reform Na+ and OH-, th...
- Mon Jan 27, 2020 6:02 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Steam graph
- Replies: 5
- Views: 284
Re: Steam graph
The graph that he showed was the heating curve for water. Other people have already explained the steam portion, so I'll talk about the graph itself. The y-axis is temperature and the x-axis is heat absorbed. When the heat added is increasing and the graph is increasing (line has a slope), then the ...
- Mon Jan 27, 2020 5:38 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Test 2
- Replies: 7
- Views: 252
Test 2
I wasn't sure which topic to put this question under, but does anyone know if test 2 is going to be cumulative or only cover material from test 1 to test 2?
- Mon Jan 27, 2020 5:25 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: delta U
- Replies: 4
- Views: 92
Re: delta U
Delta U equals work plus q. I think for this class we do not need to worry about the work involved in a calorimeter reaction/heat reaction. We only need to know the simplified version, when there is no excess work done, where delta h equals q.
- Mon Jan 27, 2020 5:01 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Calorimeter
- Replies: 3
- Views: 120
Re: Calorimeter
The system is the reaction itself, and the surroundings are the "chamber". So if the reaction is endothermic, the surroundings should lower in temperature while the system, the reaction, gains heat. If the reaction is exothermic, the surroundings should increase in temperature while the sy...
- Mon Jan 27, 2020 4:55 pm
- Forum: Phase Changes & Related Calculations
- Topic: sig figs
- Replies: 6
- Views: 239
Re: sig figs
Usually in cases like these, since 25 degrees celsius and 1 atm are constants and taken just as constants in the surroundings, you take into account the sig figs for the values that change (moles and liters), thus it being 3.
- Tue Jan 21, 2020 11:32 am
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Summary of Le Chatelier
- Replies: 5
- Views: 320
Re: Summary of Le Chatelier
Le Chatalier's principle states that, when put under stress, the reaction will shift in a way to minimize the stress. For example -adding reactants - reaction shifts to the right to form more products -adding products - reaction shifts to the left to form more reactants -removing reactants - reactio...
- Tue Jan 21, 2020 11:19 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Checking the approximation of "x"
- Replies: 4
- Views: 158
Checking the approximation of "x"
When checking the approximation of "x", we learned in class that if it is less than 5% of the initial then the approximation is okay. Is this based solely on the value of "x" or is it impacted by the coefficients of any of the reactants/products (like how we do 2x or 3x for the c...
- Fri Jan 17, 2020 12:01 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Equilibrium concentration of products
- Replies: 2
- Views: 110
Re: Equilibrium concentration of products
As long as you also have the equilibrium constant you should be able to.
- Fri Jan 17, 2020 11:56 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Acid to conjugate base
- Replies: 1
- Views: 99
Re: Acid to conjugate base
Most reactions will only lose one H atom at a time, so in one reaction I do not believe you can lose more than one H ion to become a conjugate base. However, polyprotic acids (those with multiple H ions), form conjugate bases in each successive reaction in which they lose an H ion (still within mult...
- Fri Jan 17, 2020 11:53 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Value of Kw
- Replies: 6
- Views: 173
Re: Value of Kw
At 25 degrees celsius, the value of Kw is always 1.0 x 10^-14. You can usually always assume that. If the temperature is different, the problem will usually give you the value of Kw at that temperature.
- Sun Jan 12, 2020 1:44 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Kp and Kc when you have gases and aqueous solutions
- Replies: 1
- Views: 450
Kp and Kc when you have gases and aqueous solutions
I don't know if this is even possible, but if you have a reaction with both gases and aqueous solutions and you needed to calculate the equilibrium constant, would you use Kp (ignoring aqueous solutions?) or Kc (convert partial pressure to concentration?)?
- Sun Jan 12, 2020 1:41 pm
- Forum: Administrative Questions and Class Announcements
- Topic: General HW question
- Replies: 9
- Views: 409
Re: General HW question
I believe it is graded on completion of the 5 problems.
- Sun Jan 12, 2020 1:40 pm
- Forum: Administrative Questions and Class Announcements
- Topic: HW 2
- Replies: 7
- Views: 261
Re: HW 2
I believe so, since we are still learning equilibrium.
- Sun Jan 12, 2020 1:39 pm
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Quick way
- Replies: 7
- Views: 371
Re: Quick way
If the volume decreases (pressure increases), the reaction will shift to the side with less moles of a gas. For example, if you had 3 moles of reactants and 2 moles of products and the pressure increased, the reaction would shift to the right since there are fewer moles of gas. If the volume increas...
- Sun Jan 12, 2020 1:37 pm
- Forum: Non-Equilibrium Conditions & The Reaction Quotient
- Topic: ICE tables and the quadratic equation [ENDORSED]
- Replies: 3
- Views: 249
ICE tables and the quadratic equation [ENDORSED]
I remember in high school we learned that when doing ICE tables and having a the final concentration (in the ice table) being something like 3.00 - x, we would just approximate it to 3.00 or something like that. Is there any situation we would apply that approximation to in class or are we only usin...
- Thu Dec 05, 2019 2:33 pm
- Forum: Identifying Acidic & Basic Salts
- Topic: HW 6D.11 e and f
- Replies: 1
- Views: 91
HW 6D.11 e and f
"Decide whether an aqueous solution of each of the following has a pH equal to, greater than, or less than 7.
e.) AlCl3
f.) Cu(NO3)2
I am a little confused as to why the pH in both of these problems is less than 7 and why both Cu and Al can bond with H2O.
e.) AlCl3
f.) Cu(NO3)2
I am a little confused as to why the pH in both of these problems is less than 7 and why both Cu and Al can bond with H2O.
Re: 9C 1C
The coordination compound is an anion, therefore we need to add the suffix "-ate" to the end of the transition metal name to indicate that.
- Thu Dec 05, 2019 2:16 pm
- Forum: *Titrations & Titration Calculations
- Topic: Titrations
- Replies: 1
- Views: 466
Re: Titrations
I think for titrations, since he mentioned that they were 14B and 14BL material, we will most likely just have to know the stoichiometric point and what it means (when moles of the acid = moles of the base) and whether or not the resulting solution will be basic or acidic/if the pH at the point of t...
Re: 9C 1A
We have to add the -ate to the transition metal since the coordination compound is an anion, and when we do this with iron, we use the latin name of iron (ferrum) and change it to add -ate to it.
- Thu Dec 05, 2019 2:09 pm
- Forum: Student Social/Study Group
- Topic: nomenclature for coordination compounds
- Replies: 1
- Views: 215
Re: nomenclature for coordination compounds
Chloro and chlorido are the same; chlorido is just the newer naming system, so I don't think it matters which one you use (hopefully) as they mean the same thing.
- Mon Dec 02, 2019 12:23 am
- Forum: Conjugate Acids & Bases
- Topic: Conjugate acids and bases with lewis/bronsted acids
- Replies: 3
- Views: 237
Conjugate acids and bases with lewis/bronsted acids
Do the conjugate acid/base pairs change depending on whether or not a molecule is a bronzed acid/base or lewis acid/base?
- Mon Dec 02, 2019 12:21 am
- Forum: Properties & Structures of Inorganic & Organic Bases
- Topic: Difference between inorganic and organic
- Replies: 4
- Views: 810
Re: Difference between inorganic and organic
Organic bases are bases that can donate an electron pair (usually include nitrogen). Inoragnic bases are those which dissociate into hydroxide.
- Mon Dec 02, 2019 12:20 am
- Forum: Identifying Acidic & Basic Salts
- Topic: Relative Acidity
- Replies: 2
- Views: 217
Re: Relative Acidity
Strong acids have weak bonds holding the hydrogen atom to the anion is weak (weaker, longer bond between the two). Weak bonds are usually unstable, as you can easily break them apart, which is why strong acids dissociate 100% in water. They prefer to be in a more stable state, which is with the anio...
- Mon Dec 02, 2019 12:14 am
- Forum: Calculating pH or pOH for Strong & Weak Acids & Bases
- Topic: Water as a acid or base
- Replies: 4
- Views: 307
Re: Water as a acid or base
I think so, as water is considered an amphoteric substance (can act as both an acid and a base). I believe you would only be able to classify it as specifically an acid or base when it reacts with another molecule, and not solely its own dissociation.
- Mon Dec 02, 2019 12:12 am
- Forum: Properties & Structures of Inorganic & Organic Bases
- Topic: Strength
- Replies: 4
- Views: 264
Re: Strength
Strong bases are those that are group one or two elements bonded with either hydroxide or oxide (example: NaOH, BaOH2, LiO, BeO2).
- Fri Nov 22, 2019 10:25 am
- Forum: Polarisability of Anions, The Polarizing Power of Cations
- Topic: Polarizing power of cations
- Replies: 2
- Views: 225
Polarizing power of cations
What is more important when determining the polarizing power of a cation - its size or its charge?
- Fri Nov 22, 2019 10:24 am
- Forum: Bond Lengths & Energies
- Topic: Boiling Point vs Melting Point
- Replies: 5
- Views: 647
Re: Boiling Point vs Melting Point
You're correct about the melting and boiling point increasing with stronger intermolecular forces. The difference between the two is that when melting, a substance goes from a solid to a liquid and in boiling, a substance goes from a liquid to a gas. Usually boiling requires more energy than melting.
- Fri Nov 22, 2019 10:20 am
- Forum: Dipole Moments
- Topic: Polarity with non-polar bonds
- Replies: 3
- Views: 329
Polarity with non-polar bonds
Can a molecule be polar if it only has non-polar bonds? How do lone pairs factor into the polarity of a molecule?
- Fri Nov 22, 2019 10:15 am
- Forum: Biological Examples
- Topic: EDTA
- Replies: 3
- Views: 218
Re: EDTA
EDTA is often added into solutions because it binds to the metals and thus "removes" them from reacting in the solution.
- Thu Nov 21, 2019 5:40 pm
- Forum: Interionic and Intermolecular Forces (Ion-Ion, Ion-Dipole, Dipole-Dipole, Dipole-Induced Dipole, Dispersion/Induced Dipole-Induced Dipole/London Forces, Hydrogen Bonding)
- Topic: Hydrogen Bonding Rules
- Replies: 6
- Views: 379
Re: Hydrogen Bonding Rules
Each lone pair on an N, O, of F is considered a Hydrogen bonding site.
- Fri Nov 15, 2019 11:03 am
- Forum: Interionic and Intermolecular Forces (Ion-Ion, Ion-Dipole, Dipole-Dipole, Dipole-Induced Dipole, Dispersion/Induced Dipole-Induced Dipole/London Forces, Hydrogen Bonding)
- Topic: tetrahedral/triangular pyramidal
- Replies: 2
- Views: 125
Re: tetrahedral/triangular pyramidal
This is where lone pairs come into play. A molecule with four regions of electron density (lone pairs and bonded atoms) is considered tetrahedral, but if there are 3 bonded regions and one lone pair, the shape is trigonal pyramidal. Basically, when you count the regions of electron density, that giv...
- Fri Nov 15, 2019 10:57 am
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Polar vs Nonpolar molecules
- Replies: 4
- Views: 193
Re: Polar vs Nonpolar molecules
A little "cheat" you can follow is that all of the VSEPR models that do not have lone pairs (linear, trigonal planar, tetrahedral, trigonal bipyramidal, octahedral) and where all of the bonds are the same (central atom bonded to the same type of atom/atoms of the same element, ie. CCl4, CH...
- Fri Nov 15, 2019 10:51 am
- Forum: *Liquid Structure (Viscosity, Surface Tension, Liquid Crystals, Ionic Liquids)
- Topic: Viscosity/Surface Tension
- Replies: 7
- Views: 1737
Re: Viscosity/Surface Tension
When molecules have stronger IMF, they are more strongly attracted to each other and therefore do not have as much motion as molecules that are not as attracted to each other. Therefore, molecules with a stronger IMF will have a higher viscosity than molecules with low IMF. The same thing applies to...
- Fri Nov 15, 2019 10:44 am
- Forum: Interionic and Intermolecular Forces (Ion-Ion, Ion-Dipole, Dipole-Dipole, Dipole-Induced Dipole, Dispersion/Induced Dipole-Induced Dipole/London Forces, Hydrogen Bonding)
- Topic: Does dipole-dipole force only exist among polar molecules?
- Replies: 9
- Views: 1310
Re: Does dipole-dipole force only exist among polar molecules?
I think so. In order to have a dipole-dipole force, you must have polar bonds within a molecule that don't cancel out (essentially the molecule is polar). Dipole-induced dipole, however, exists among polar and non-polar molecules.
- Fri Nov 15, 2019 10:42 am
- Forum: Polarisability of Anions, The Polarizing Power of Cations
- Topic: Will polarizability/polarizing power be on test 2?
- Replies: 3
- Views: 305
Will polarizability/polarizing power be on test 2?
Since it wasn't on the midterm, will polarizing power/polarizability be included on test 2?
- Fri Nov 08, 2019 12:23 am
- Forum: Lewis Structures
- Topic: Drawing Lewis Structures
- Replies: 18
- Views: 694
Re: Drawing Lewis Structures
As mentioned above, the overall charges should add up to the charge of the molecule. However, you usually want more of the atoms to have a formal charge of zero, so if you can make a structure in which the overall formal charges add up to the charge of the molecule and have the atoms where it's poss...
- Thu Nov 07, 2019 11:13 pm
- Forum: Lewis Structures
- Topic: Exceptions to Octet Rule
- Replies: 7
- Views: 417
Re: Exceptions to Octet Rule
Yes, elements in period 3 and on are exceptions to the octet rule (can have an expanded octet) because they have a d-orbital that is not filled and that can accept electrons (this is because the d orbital starts existing/being found when n=3, as in you can have a 3d orbital but not a 2d or 1d orbita...
- Thu Nov 07, 2019 11:03 pm
- Forum: Empirical & Molecular Formulas
- Topic: Empirical formula
- Replies: 3
- Views: 323
Re: Empirical formula
When you're given the amount of molecules produced (they usually won't give you percentages of molecules since the molecules represent the products), with molecules being something like H20 or CO2, you convert the grams of the molecules to moles of the molecule. Then, you need to use the molar ratio...
- Thu Nov 07, 2019 10:51 pm
- Forum: Empirical & Molecular Formulas
- Topic: shortcut?
- Replies: 2
- Views: 235
Re: shortcut?
When you are already given the calculated molar mass of the compound, you just need to multiply the percentages by that molar mass to get the grams of each element and then divide that by the molar mass of the element to get the moles. You do not need to divide each mole of each element by the small...
- Thu Nov 07, 2019 10:39 pm
- Forum: Bond Lengths & Energies
- Topic: dissociation energy
- Replies: 6
- Views: 414
Re: dissociation energy
I believe the only thing we need to know about bond dissociation energy for now is that stronger bonds have a larger dissociation energy (requires more energy to break them) and weaker bonds have a smaller dissociation energy (requires less energy to break them).
- Fri Nov 01, 2019 10:40 am
- Forum: Electronegativity
- Topic: Electronegativity on Test
- Replies: 7
- Views: 259
Re: Electronegativity on Test
I think you might need to know if a bond is going to be ionic or covalent based on the electronegativities (above 2 = ionic, below 1.5 = covalent) along with the trends in electronegativity. But since we haven't really done ay problems calculating exact electronegativities yet, I doubt that'll be on...
- Fri Nov 01, 2019 10:38 am
- Forum: Formal Charge and Oxidation Numbers
- Topic: Formal Charges on Atoms Summed in Ions?
- Replies: 6
- Views: 205
Re: Formal Charges on Atoms Summed in Ions?
If the molecule does not have a charge associated to it (a charge like 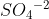 ), then the formal charges should cancel out to zero. In the example of the molecule I mentioned the overall formal charges of the molecule should equal -2, since the molecule has a charge of -2.
), then the formal charges should cancel out to zero. In the example of the molecule I mentioned the overall formal charges of the molecule should equal -2, since the molecule has a charge of -2.
- Thu Oct 31, 2019 5:46 pm
- Forum: DeBroglie Equation
- Topic: Converting mass to kilograms for de broglie
- Replies: 7
- Views: 294
Converting mass to kilograms for de broglie
If a problem wants you to find the wavelength of an ion or an atom given the speed its traveling and no other information, how do you get the mass of the singular atom? If it was potassium for example, would you use the molar mass of potassium, change it to kilograms, and then divide by avogadros nu...
- Thu Oct 31, 2019 5:44 pm
- Forum: DeBroglie Equation
- Topic: When to use the de Broglie equation?
- Replies: 5
- Views: 298
When to use the de Broglie equation?
Do you only use this equation to find the wavelength or velocity of something that is not light (electrons, atoms, objects, etc.)?
- Thu Oct 31, 2019 5:36 pm
- Forum: Properties of Light
- Topic: Which equations can you only use for light?
- Replies: 2
- Views: 92
Which equations can you only use for light?
Can you only use the equations c=wavelength times frequency and energy = h times frequency for light (and not for atoms for example)?
- Thu Oct 24, 2019 1:53 pm
- Forum: Wave Functions and s-, p-, d-, f- Orbitals
- Topic: Energy of spdf orbitals
- Replies: 11
- Views: 399
Re: Energy of spdf orbitals
705198479 wrote:how do I know how many bonds or "dots" go on the ion in the lewis structures
The number of dots on an atom is the same as the number of valence electrons that the atom has. So for carbon, for example, you would draw four dots as it has four valence electrons.
- Thu Oct 24, 2019 1:51 pm
- Forum: Wave Functions and s-, p-, d-, f- Orbitals
- Topic: HW 1D.23
- Replies: 4
- Views: 334
HW 1D.23
Can someone explain to me why the answer for part D is 4?
- Thu Oct 24, 2019 1:50 pm
- Forum: Resonance Structures
- Topic: bond lengths
- Replies: 4
- Views: 170
Re: bond lengths
Adding on to the other answers, the molecule has resonance, and the more resonance structures a molecule has, the more stable it is.
- Thu Oct 24, 2019 1:42 pm
- Forum: Ionic & Covalent Bonds
- Topic: Ionic v.covalent bonds
- Replies: 7
- Views: 369
Re: Ionic v.covalent bonds
You're correct! You can also compare the electronegativities of the of the atoms involved in the bond. If the difference between their electronegativities is greater than 1.6, then the bond is ionic.
- Thu Oct 24, 2019 1:40 pm
- Forum: Ionic & Covalent Bonds
- Topic: Cation
- Replies: 23
- Views: 1775
Re: Cation
Also, cations are smaller than their originating atom because they have lost an electron, and thus the attraction between the fewer electrons and the positive charge from the nucleus is greater, resulting in a smaller ion (more attraction). Anions are larger than their originating atom because they ...
- Fri Oct 18, 2019 10:22 am
- Forum: Properties of Electrons
- Topic: Delta
- Replies: 3
- Views: 182
Re: Delta
I think when an e- goes from n=3 to n=1 the change in energy is negative because its releasing energy (going from a higher energy state to a lower one, and the change in energy is measured as En(final) - En(initial)). Going from n=1 to n=3 should be a positive change in energy since you have to abso...
- Fri Oct 18, 2019 10:20 am
- Forum: *Shrodinger Equation
- Topic: Math Function
- Replies: 4
- Views: 209
Re: Math Function
From what I understand, when we mention "math function" it relates to the probability of finding and e- in a certain area (at least for the Schrodinger equation), which is represented by the math function (sin or cos squared, where the x-intercept represents a zero probability of finding a...
- Fri Oct 18, 2019 10:06 am
- Forum: Photoelectric Effect
- Topic: Photoelectric Effect
- Replies: 6
- Views: 325
Re: Photoelectric Effect
I believe so. The electron would not have any velocity (through the kinetic energy equation KE = 1/2 mv^2) to fully remove itself from the metal, but it is considered "ejected".
- Thu Oct 17, 2019 7:25 pm
- Forum: Wave Functions and s-, p-, d-, f- Orbitals
- Topic: Drawing Orbitals
- Replies: 5
- Views: 282
Re: Drawing Orbitals
I think he mentioned in class that we will not actually have to draw out the orbitals and that they're just a visual tool.
- Tue Oct 15, 2019 4:03 pm
- Forum: DeBroglie Equation
- Topic: HW 1b.15 part c
- Replies: 2
- Views: 105
HW 1b.15 part c
I am confused as to why, for part c when it asks for the wavelength of the radiation, you can't just use the relation that c = frequency times wavelength, seeing that they give you the frequency of the radiation in part b? Why do we have to use the equation we learned in class instead?
- Thu Oct 10, 2019 3:16 pm
- Forum: Limiting Reactant Calculations
- Topic: Test 1 Calculator
- Replies: 6
- Views: 404
Re: Test 1 Calculator
I guess graphing calculators allow you to store equations and they usually are programmable, so it's there way of preventing us from cheating by storing equations they want us to memorize.
- Thu Oct 10, 2019 3:14 pm
- Forum: Empirical & Molecular Formulas
- Topic: Converting from grams to percentage
- Replies: 11
- Views: 7664
Converting from grams to percentage
In class when we completed the Vitamin C problem, I remember that we converted the grams of each element into percentage using the total grams of vitamin c. In other problems (such as F13), it is not required to convert to percentage to do the problem. I guess my question is when do we have to conve...
- Thu Oct 10, 2019 3:07 pm
- Forum: Accuracy, Precision, Mole, Other Definitions
- Topic: # of molecules and formula units?
- Replies: 5
- Views: 365
# of molecules and formula units?
Are "number of molecules" and "formula units" considered the same unit? As in, do we use avogadro's number to get the answer when its required to be in those units?
- Thu Oct 10, 2019 3:04 pm
- Forum: Properties of Electrons
- Topic: Understanding Balmer & Lyman Series
- Replies: 3
- Views: 194
Re: Understanding Balmer & Lyman Series
The Balmer series refers to the UV light emissions. Essentially, UV light is created whenever an electron jumps to the energy level of n=1. n refers to the energy levels. It does not matter whether an electron jumped from n=2 to n=1or n=4 to n=4 - whenever an electron jumps to n=1, the light emitted...
- Thu Oct 10, 2019 2:59 pm
- Forum: Accuracy, Precision, Mole, Other Definitions
- Topic: hw question E23?
- Replies: 3
- Views: 262
Re: hw question E23?
To calculate the moles of an ion in a molecular compound, you see how many moles of that are present in the compound and use that number as the multiplication factor. For example, to see how many moles of Na+ are in Na2CO3, you would find the total number of moles of Na2CO3, and you know that for ea...
- Tue Oct 01, 2019 4:03 pm
- Forum: Accuracy, Precision, Mole, Other Definitions
- Topic: Accuracy and Precision [ENDORSED]
- Replies: 4
- Views: 265
Accuracy and Precision [ENDORSED]
Will we have to know how to calculate accuracy/precision (I vaguely remember something about a number plus or minus another number but do not remember how to get to that point)?
- Tue Oct 01, 2019 3:59 pm
- Forum: Accuracy, Precision, Mole, Other Definitions
- Topic: G.25 Dilution
- Replies: 4
- Views: 214
Re: G.25 Dilution
Starting from the beginning you can use the MiVi = MfVf equation. Thus, you have the original molarity of the solution times 0.01 L (convert milliliters to liters) = the new/unknown concentration (can give it variable x) times 0.09 L (90 ml converted to liters). Thus you find the molarity by finding...
- Tue Oct 01, 2019 3:40 pm
- Forum: Limiting Reactant Calculations
- Topic: Finding limiting reagent
- Replies: 6
- Views: 822
Re: Finding limiting reagent
The process that helps me the most with the mole to mole ratio is this. 1. Convert grams of reactant to moles of the reactant using molar mass. 2. Convert the moles of the reactant to moles of the reactant using the stoichiometric coefficients given in the balanced chemical equation (for example, if...
- Tue Oct 01, 2019 3:33 pm
- Forum: Molarity, Solutions, Dilutions
- Topic: Conversion for the example dilution problem done in class on Monday
- Replies: 2
- Views: 108
Re: Conversion for the example dilution problem done in class on Monday
Usually the problem will specify which units to leave the answer in. Just make sure to convert to liters when doing the molarity calculations! :)
- Sun Sep 29, 2019 9:10 pm
- Forum: Significant Figures
- Topic: Sig Figs in E21 a/b
- Replies: 3
- Views: 161
Re: Sig Figs in E21 a/b
In the first problem, since there are 3 sig figs in 10.0 (the last zero still counts as it shows to which decimal it is accurate), you need your answer to have 3 sig figs (0.0981, as the zero after the decimal and before the 9 is not significant). On the second one I'm not actually sure because ther...

