Search found 99 matches
- Mon Mar 08, 2021 3:17 pm
- Forum: Student Social/Study Group
- Topic: Points needed to pass?
- Replies: 76
- Views: 7547
Re: Points needed to pass?
You would need at least 200 points to pass (out of a total of 400 points) since a 50% or higher is a passing grade for this course. Otherwise, standard grading metrics apply.
- Mon Mar 08, 2021 3:15 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Week 9 Sapling
- Replies: 11
- Views: 637
Re: Week 9 Sapling
Yes, there is a Sapling assignment for both weeks 9 and 10, but to compensate for the final exam being on Sunday, the Sapling assignment is due Friday night instead of the usual Sunday night.
- Mon Mar 08, 2021 3:13 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Final Exam Time
- Replies: 47
- Views: 2388
Re: Final Exam Time
The final exam will be on Sunday from 9:30 A.M. PST to 11:00 A.M. PST, and a thermodynamics review session will be on Saturday from 8:00 A.M. PST to 10:00 A.M. PST.
- Mon Mar 08, 2021 3:09 pm
- Forum: Student Social/Study Group
- Topic: How do you deal with burnout?
- Replies: 144
- Views: 15128
Re: How do you deal with burnout?
Personally, to deal with burnout, I generally try to plan out my week so that I can distribute my workload more efficiently. In addition, setting time to exercise and relax certainly does wonders as this allows for better and mental health as well as a break of school.
- Mon Mar 08, 2021 3:00 pm
- Forum: Student Social/Study Group
- Topic: Note Taking
- Replies: 145
- Views: 16454
Re: Note Taking
Personally, in my own opinion, the most effective way to take notes in this class is to handwrite them, since for me handwriting my notes help me retain the information better than other methods such as typing them
- Sun Mar 07, 2021 11:58 pm
- Forum: Method of Initial Rates (To Determine n and k)
- Topic: Slowest step
- Replies: 38
- Views: 1627
Re: Slowest step
The slowest step is considered the rate determining state because it takes the most time and the overall reaction will not finish until the slowest step is complete.
- Sun Mar 07, 2021 11:55 pm
- Forum: General Rate Laws
- Topic: Factors Affecting k
- Replies: 83
- Views: 5149
Re: Factors Affecting k
Yes, the rate constant (k) of reaction can change by a change in temperature only.
- Sun Mar 07, 2021 11:54 pm
- Forum: General Rate Laws
- Topic: Intermediates
- Replies: 17
- Views: 1502
Re: Intermediates
Intermediates cannot be in the rate law because they cancel out on both sides of the chemical equation.
- Sun Mar 07, 2021 11:52 pm
- Forum: Kinetics vs. Thermodynamics Controlling a Reaction
- Topic: Instantaneous Rate
- Replies: 41
- Views: 2235
Re: Instantaneous Rate
As the reaction proceeds, the instantaneous rate decreases over time.
- Sun Mar 07, 2021 11:50 pm
- Forum: Kinetics vs. Thermodynamics Controlling a Reaction
- Topic: What was your favorite chem topic?
- Replies: 137
- Views: 10937
Re: What was your favorite chem topic?
Chemical equilibrium and acids and bases.
- Sun Feb 28, 2021 9:01 pm
- Forum: Balancing Redox Reactions
- Topic: Oxidation Numbers
- Replies: 30
- Views: 1337
Re: Oxidation Numbers
No, you do not need to memorize the oxidation numbers - just a remember a few of the more general ones (like H and O are usually +1 and -2 respectively) and you should be fine - often times you can find the rest of the oxidation numbers that you do not already know from this information.
- Sun Feb 28, 2021 9:00 pm
- Forum: Balancing Redox Reactions
- Topic: Determining Phases
- Replies: 28
- Views: 1069
Re: Determining Phases
Just use the phase states from the original equation, since they should not really change, and when you add water and ions they are in liquid and aqueous states respectively.
- Sun Feb 28, 2021 8:57 pm
- Forum: Balancing Redox Reactions
- Topic: Balancing Reactions
- Replies: 22
- Views: 1088
Re: Balancing Reactions
Order doesn't really matter - as long as your reactants and products are correct and in their respective places before or after the chemical equation sign then you should be fine.
- Sun Feb 28, 2021 8:56 pm
- Forum: Balancing Redox Reactions
- Topic: states of matter
- Replies: 58
- Views: 2379
Re: states of matter
Yes, states of matter can cause an equation on Sapling to be wrong, so make sure to include the correct phase states.
- Sun Feb 28, 2021 8:54 pm
- Forum: Balancing Redox Reactions
- Topic: Sapling #1 Glitch
- Replies: 16
- Views: 843
Re: Sapling #1 Glitch
Try refreshing the page or maybe clearing your cookies - hope this helps!
- Mon Feb 15, 2021 1:46 pm
- Forum: Concepts & Calculations Using Second Law of Thermodynamics
- Topic: Adding Equations
- Replies: 16
- Views: 811
Re: Adding Equations
Yes, when we are adding equations, the same rules that apply to delta H also apply to delta S. This is because enthalpy and entropy are both state functions, so only the initial and final values matter, but not the path taken to get there.
- Mon Feb 15, 2021 1:44 pm
- Forum: Concepts & Calculations Using Second Law of Thermodynamics
- Topic: Entropy: kJ or J?
- Replies: 30
- Views: 1607
Re: Entropy: kJ or J?
It depends on the context of the question, so use whichever unit / conversion that more relevant and applicable to the problem. Generally, entropy is solved in J/K though.
- Mon Feb 15, 2021 1:42 pm
- Forum: Concepts & Calculations Using Second Law of Thermodynamics
- Topic: Entropy definition
- Replies: 37
- Views: 2531
Re: Entropy definition
Entropy is the measure of the disorder or randomness of a system. It is usually denoted with S.
- Mon Feb 15, 2021 1:41 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: temperature
- Replies: 32
- Views: 1333
Re: temperature
It depends on the context of the problem. Use whatever temperature scale that is relevant to the question. In general, when doing calculations for thermodynamics, Kelvin is usually used at a greater frequency than Celsius, but whichever one is applicable to the problem is the one you should use.
- Mon Feb 15, 2021 1:38 pm
- Forum: Calculating Standard Reaction Entropies (e.g. , Using Standard Molar Entropies)
- Topic: Gas Entropy
- Replies: 14
- Views: 872
Re: Gas Entropy
Yes, a gas become more orderly when it liquefies. Yes, its entropy changes. Since the transition from gas state to liquid state is more "orderly," entropy decreases as its disorder decreases.
- Sun Feb 14, 2021 11:19 pm
- Forum: Thermodynamic Systems (Open, Closed, Isolated)
- Topic: Work on a system
- Replies: 27
- Views: 1125
Re: Work on a system
An example where work is done on the system, resulting in a positive value for work, would be compressing the system.
- Sun Feb 14, 2021 11:18 pm
- Forum: Thermodynamic Systems (Open, Closed, Isolated)
- Topic: R Constant
- Replies: 91
- Views: 6215
Re: R Constant
The R value you use is the one that cancels out the rest of your units - they are all equivalent, just in different units.
- Sun Feb 14, 2021 11:17 pm
- Forum: Thermodynamic Systems (Open, Closed, Isolated)
- Topic: Closed vs Isolated System
- Replies: 30
- Views: 1363
Re: Closed vs Isolated System
The key difference between a closed and an isolated system is that a closed system can exchange energy with its surroundings, while an isolated system cannot exchange anything with its surroundings. An example of a closed system would be a piston system and an example of an isolated system would be ...
- Sun Feb 14, 2021 11:14 pm
- Forum: Thermodynamic Systems (Open, Closed, Isolated)
- Topic: Entropy
- Replies: 27
- Views: 1155
Re: Entropy
Entropy is a measure of the chaos in a system. In terms of how important it is to know for the next midterm, knowing how it works and understanding its equations might help.
- Sun Feb 14, 2021 11:11 pm
- Forum: Thermodynamic Systems (Open, Closed, Isolated)
- Topic: Free Energy
- Replies: 49
- Views: 2083
Re: Free Energy
A state function is a function that does not depend on the path that is taken to get the final value. Free energy is a state function because only the initial and final values matter - the path taken does not, however.
- Sun Feb 07, 2021 10:44 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Negative Sign
- Replies: 16
- Views: 590
Re: Negative Sign
The side of the equation the negative sign goes for is the side that is exothermic (q is negative).
- Sun Feb 07, 2021 10:42 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Extensive Properties
- Replies: 10
- Views: 479
Re: Extensive Properties
Extensive properties depend on the amount of a particular substance that we are dealing with.
- Sun Feb 07, 2021 10:36 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: q and internal energy
- Replies: 8
- Views: 384
Re: q and internal energy
We know when q = delta U (internal energy) when w is 0 and thus delta V is 0.
- Sun Feb 07, 2021 10:33 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Can heat capacities be negative?
- Replies: 52
- Views: 13935
Re: Can heat capacities be negative?
No, I don't think heat capacities be negative - they can only be positive because it's how much energy is heat is gained, so it is endothermic.
- Sun Feb 07, 2021 10:29 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: HW 14
- Replies: 13
- Views: 1678
Re: HW 14
We use the initial volume, so we use V1 for PV = nRT.
- Sun Jan 31, 2021 9:20 pm
- Forum: Phase Changes & Related Calculations
- Topic: when to assume x is insignificant
- Replies: 86
- Views: 7107
Re: when to assume x is insignificant
It is fine or restricted to assume x is so insignificant we can keep it out when it is less than 5% of the initial concentration.
- Sun Jan 31, 2021 9:17 pm
- Forum: Phase Changes & Related Calculations
- Topic: Vapor vs gas
- Replies: 121
- Views: 11290
Re: Vapor vs gas
No, there is not a difference between vapor and gas - they are the same thing and are considered interchangeable with each other and vice versa.
- Sun Jan 31, 2021 9:15 pm
- Forum: Phase Changes & Related Calculations
- Topic: H and q
- Replies: 47
- Views: 1688
Re: H and q
Heat is denoted with q and enthalpy is denoted with H, specifically.
- Sun Jan 31, 2021 9:14 pm
- Forum: Phase Changes & Related Calculations
- Topic: Define Phase Change
- Replies: 78
- Views: 5306
Re: Define Phase Change
A phase change specifically means when a substance transitions from one phase to another. These phases include solid, liquid, gas, plasma, etc. These phase transitions are completely reversible and do not change the component of the substance - just its state.
- Sun Jan 31, 2021 9:11 pm
- Forum: Phase Changes & Related Calculations
- Topic: Endothermic v. Exothermic
- Replies: 139
- Views: 14032
Re: Endothermic v. Exothermic
Yes, endothermic reactions will always have a positive delta H, and yes, exothermic reactions will always have a negative delta H. There are no exceptions to this rule.
- Sun Jan 24, 2021 4:35 pm
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Increase in Pressure
- Replies: 31
- Views: 807
Re: Increase in Pressure
When finding out which side has the least amount of moles, you only include gases, but not aqueous solutions.
- Sun Jan 24, 2021 4:33 pm
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Increasing pressure
- Replies: 23
- Views: 933
Re: Increasing pressure
The concentration of the reactants and products remain the same when inert gas is added because inert gases are not chemically reactive, so they will not react with the reactants or products and thus their concentrations will remain the same.
- Sun Jan 24, 2021 4:30 pm
- Forum: Non-Equilibrium Conditions & The Reaction Quotient
- Topic: Ka correlation to strength of an acid
- Replies: 30
- Views: 2289
Re: Ka correlation to strength of an acid
The relationship between the Ka and how strong an acid is the larger the Ka the stronger the acid.
- Sun Jan 24, 2021 4:28 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: How to find the H+ from pH
- Replies: 12
- Views: 523
Re: How to find the H+ from pH
You find the H+ concentration from the pH from just taking 10 to the power of the negative pH.
- Sun Jan 24, 2021 4:16 pm
- Forum: Ideal Gases
- Topic: Inverse Kc [ENDORSED]
- Replies: 41
- Views: 2110
Re: Inverse Kc [ENDORSED]
We have to use the inverse of Kc or any K when we are considering the reverse reaction.
- Sun Jan 17, 2021 8:13 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Sapling Question 4
- Replies: 5
- Views: 360
Re: Sapling Question 4
For this problem, you would have to set up an ICE table just like any other equilibrium - related question. Once you have solved for x and consequently the equilibrium pressures, you would have to add all of them to get the total pressure at equilibrium.
- Sun Jan 17, 2021 8:10 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Sapling Week 1 #9
- Replies: 11
- Views: 788
Re: Sapling Week 1 #9
The changes in x should be +x for both N2(g) and O2(g) and -2x for 2NO(g). This is because since you are adding products, the equilibrium reaction will shift to the left to create more reactants to compensate for the change, and thus reactants will increase and products will decrease.
- Sun Jan 17, 2021 8:06 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Sapling Week 1 #3
- Replies: 9
- Views: 331
Re: Sapling Week 1 #3
For this question, you would have to set up an ICE table. One tip is that you can just take the square root the Kc formula and the value for Kc to solve for x. Although this is a shortcut, I would not recommend using this and instead solve the entire quadratic formula since taking the square root of...
- Sun Jan 17, 2021 8:02 pm
- Forum: Student Social/Study Group
- Topic: Chemistry Community Posts
- Replies: 22
- Views: 953
Re: Chemistry Community Posts
I would double - check with your TA, but I would recommend doing 5 Chemistry Community posts per week for the duration of the 10 weeks. Not only does this minimize risk in terms of getting the 50 points for your 50 posts total, but it will also help keep you on track with the material.
- Sun Jan 17, 2021 7:58 pm
- Forum: Student Social/Study Group
- Topic: Midterms During Lecture
- Replies: 44
- Views: 1995
Re: Midterms During Lecture
Yes, exams are being held during lecture this quarter - this includes both midterm and final exams. This information comes from both the Syllabus and the Exam Schedule. The midterms are 50 minutes and the final is 1 hour and 30 minutes.
- Sun Jan 10, 2021 9:31 pm
- Forum: Ideal Gases
- Topic: PV=nRT and concentration
- Replies: 27
- Views: 1596
Re: PV=nRT and concentration
Molarity (or concentration) is represented in moles / volume, which is the respective unit of measure for molarity (or concentration).
- Sun Jan 10, 2021 9:26 pm
- Forum: Administrative Questions and Class Announcements
- Topic: How to find my posts
- Replies: 163
- Views: 166173
Re: How to find my posts
You can find your posts my clicking on "Quick Links" and then "Your Posts." Sometimes, there is some discrepancy with the number of times you've actually posted and the displayed number, so be sure to double - check and refresh the page often.
- Sun Jan 10, 2021 9:23 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Registering/Creating Your Chemistry Community Account
- Replies: 40
- Views: 100852
Re: Registering/Creating Your Chemistry Community Account
Yes, it is 5 posts per week for a total of 50 posts (50 points). I personally have my display name as my full name and discussion section, but your account is linked to your UID so that grading our posts would be easier.
- Sun Jan 10, 2021 9:20 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Accessing the E-textbook [ENDORSED]
- Replies: 125
- Views: 32056
Re: Accessing the E-textbook [ENDORSED]
Yes, what is due each week are 5 posts on Chemistry Community and the Sapling assignments. Both are 5 points each for the 10 weeks, for a total of 100 points for both combined.
- Wed Dec 04, 2019 6:19 pm
- Forum: Student Social/Study Group
- Topic: Preparing for the final
- Replies: 25
- Views: 1265
Re: Preparing for the final
Try to go to as many TA review sessions as possible, and practice as many problems as you possibly can.
- Wed Dec 04, 2019 6:16 pm
- Forum: Bronsted Acids & Bases
- Topic: CaO
- Replies: 10
- Views: 1564
Re: CaO
CaO is a strong base because it fully dissociates to give ions in solution.
- Wed Dec 04, 2019 6:14 pm
- Forum: Lewis Acids & Bases
- Topic: diff b/w lewis acid and base
- Replies: 12
- Views: 702
Re: diff b/w lewis acid and base
Lewis acids are electron - pair acceptors and Lewis bases are electron - pair donors.
- Wed Dec 04, 2019 6:12 pm
- Forum: Lewis Acids & Bases
- Topic: Definition
- Replies: 7
- Views: 573
Re: Definition
The definitions of a Lewis acid and base that Dr. Lavelle wants are electron - pair acceptor and electron - pair donor, respectively.
- Wed Dec 04, 2019 6:09 pm
- Forum: Lewis Acids & Bases
- Topic: Oxoacids
- Replies: 6
- Views: 426
Re: Oxoacids
Oxoacids are acids that contain oxygen. To be more specific, oxoacids are acids that contain oxygen, contain at least one other element, have at least one hydrogen atom bonded to oxygen, and form an ion by the loss of one or more protons in solution.
- Sun Dec 01, 2019 11:55 pm
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: Cis vs Trans
- Replies: 22
- Views: 1735
Re: Cis vs Trans
The difference between the terms cis and trans is that cis means polar and trans means nonpolar.
- Sun Dec 01, 2019 11:50 pm
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: brackets
- Replies: 13
- Views: 676
Re: brackets
What the brackets signify when they are around the compound is a coordination compound.
- Sun Dec 01, 2019 11:48 pm
- Forum: Naming
- Topic: Final Exam?
- Replies: 20
- Views: 1142
Re: Final Exam?
The final exam is cumulative, so we can just base off what we need to know by using the outlines.
- Sun Dec 01, 2019 11:47 pm
- Forum: Naming
- Topic: Roman Numeral
- Replies: 13
- Views: 915
Re: Roman Numeral
The roman numerals represent the oxidation number of the metal ion.
Re: Oxidation
An oxidation number is a number associated with the charge that an atom would have if the compound was composed of ions.
- Sun Nov 24, 2019 11:44 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Drawing molecules
- Replies: 7
- Views: 484
Re: Drawing molecules
I personally don't think we will ever have to draw molecules in such a way that was similar to what was on the test - it is most likely for a future chemistry class (nonetheless, you will be better off safe than sorry if you prepared so).
- Sun Nov 24, 2019 11:39 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Lone Pairs on Cenrtral Atom
- Replies: 11
- Views: 648
Re: Lone Pairs on Cenrtral Atom
Lone pairs around a central atom affect the bond angles in a molecule by decreasing them due to the repulsion between electrons.
- Sun Nov 24, 2019 11:34 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: AXE Format
- Replies: 34
- Views: 1244
Re: AXE Format
Whenever there's only one X or one E, we do not have to write a subscript of "1" - we just write "X" and "E," respectively.
- Sun Nov 24, 2019 11:31 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Final
- Replies: 10
- Views: 567
Re: Final
Yes, the final is cumulative.
- Sun Nov 24, 2019 11:30 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: melting point
- Replies: 8
- Views: 644
Re: melting point
If one bond were to be broken, what happens to the melting point is that it decreases.
- Sat Nov 16, 2019 4:40 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Polar vs. Nonpolar
- Replies: 12
- Views: 813
Re: Polar vs. Nonpolar
It is generally not recommended to look at a molecule's geometry / shape when determining if it's polar or non polar. It is best to use the VSEPR model and calculate the actual dipole moments to see if they add up to a net value of 0. If they do, the molecule is non polar, and if they do not, the mo...
- Sat Nov 16, 2019 4:32 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: All VSEPR structures or just ones from class?
- Replies: 11
- Views: 645
Re: All VSEPR structures or just ones from class?
We should know all of the ones on the chart instead of only the ones Dr. Lavelle went over in class for Test 2. It's better to be safe than sorry.
- Sat Nov 16, 2019 4:30 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Determining if a molecule is polar or non polar
- Replies: 9
- Views: 920
Re: Determining if a molecule is polar or non polar
When determining if a molecule is polar or non polar on the test, it is not expected of us to know the electronegativity values of the elements in the given molecules. Just know the periodic trends for electronegativity and you should be fine.
- Sat Nov 16, 2019 4:25 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: How to study for VSEPR?
- Replies: 9
- Views: 718
Re: How to study for VSEPR?
In terms of studying for VSEPR for Test 2, just do as much practice problems as you can - the more the better. In terms of "good" practice problem suggestions, start by completing all the practice problems in the Molecular Shape and Structure unit outline (although only 5 are required for ...
- Sat Nov 16, 2019 4:17 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: test 2
- Replies: 13
- Views: 734
Re: test 2
We do not have to know hybridization for Test 2 (as stated by Dr. Lavelle in class). However, pi and sigma bonds will be on Test 2, for which Dr. Lavelle will cover those topics in class on Monday. Essentially, Test 2 will cover all the material covered in class up until Monday after the midterm exam.
- Sat Nov 09, 2019 12:04 am
- Forum: Resonance Structures
- Topic: Resonance Structures
- Replies: 18
- Views: 1136
Re: Resonance Structures
Resonance structures are two or more forms of a molecule where the chemical connectivity is the same but the electrons are distributed differently around the structure.
- Sat Nov 09, 2019 12:03 am
- Forum: Electronegativity
- Topic: Trend of Electronegativity
- Replies: 22
- Views: 2125
Re: Trend of Electronegativity
Electronegativities generally increase from left to right across a period. This is due to an increase in nuclear charge. Electronegativities generally decrease from top to bottom within a group due to the larger atomic size.
- Sat Nov 09, 2019 12:02 am
- Forum: Formal Charge and Oxidation Numbers
- Topic: Formal Charge Equation?
- Replies: 12
- Views: 565
Re: Formal Charge Equation?
Yes, both depend on how you count the shared bond. The first equation treats the number of bonds as how many "lines" you draw as bonds, while the second equation treats the number of bonds as the amount of valence electrons that are shared in total between an atom and another respective at...
- Fri Nov 08, 2019 11:55 pm
- Forum: Formal Charge and Oxidation Numbers
- Topic: central atom
- Replies: 21
- Views: 1031
Re: central atom
Yes, we want to make it so that the central atom has a formal charge of zero, or as close to zero as it can get - that would be the ideal situation.
- Fri Nov 08, 2019 11:53 pm
- Forum: Formal Charge and Oxidation Numbers
- Topic: Showing Work
- Replies: 6
- Views: 300
Re: Showing Work
We should write out the entire equation for formal charge on the midterm instead of just calculating it in our head and writing the formal charge by the element because not only will writing out the entire equation for formal charge make it easier to organize both your thoughts and work, but it will...
- Thu Oct 31, 2019 5:47 pm
- Forum: Lewis Structures
- Topic: valence electrons
- Replies: 4
- Views: 364
Re: valence electrons
The trends for valence electrons are as follows: in a period, the number of valence electrons increases as we move from left to right side and in a group, the number of valence electrons remains the same.
- Thu Oct 31, 2019 5:44 pm
- Forum: Lewis Structures
- Topic: Drawing lewis structures
- Replies: 8
- Views: 267
Re: Drawing lewis structures
Since this topic has not been covered in - depth yet in the lectures, you shouldn't worry too much about it. But, in general, it is better to be safe than sorry, so when drawing Lewis structures on tests, you should angle the bonds in the correct ways.
- Thu Oct 31, 2019 5:39 pm
- Forum: Ionic & Covalent Bonds
- Topic: What is the Octet Rule?
- Replies: 5
- Views: 343
Re: What is the Octet Rule?
The concept of the octet rule is that elements tend to bond in such a way that each atom has eight electrons in its valence shell, giving it the same electronic configuration as a noble gas.
- Thu Oct 31, 2019 5:37 pm
- Forum: Ionic & Covalent Bonds
- Topic: Lewis structure
- Replies: 3
- Views: 183
Re: Lewis structure
When we draw the Lewis structure, we know which atom is in the middle in that usually the central atom will be the one that has the most unpaired valence electrons - usually it is also the least electronegative.
- Thu Oct 31, 2019 5:33 pm
- Forum: Ionic & Covalent Bonds
- Topic: Homework for Week 5
- Replies: 8
- Views: 334
Re: Homework for Week 5
For homework this week, we can do either quantum to review or chemical bonding. I personally did chemical bonding for homework, but it doesn't really matter to your designated TA as long as the material has been recently covered in lectures.
- Sat Oct 26, 2019 10:45 pm
- Forum: Electron Configurations for Multi-Electron Atoms
- Topic: Shorthand Notation
- Replies: 4
- Views: 255
Re: Shorthand Notation
For both the midterm and future tests, we should write the whole electron configuration instead of the shorthand notation because not only will you be more safe than sorry, you will gain better understanding of how to write electron configurations accurately.
- Sat Oct 26, 2019 10:35 pm
- Forum: Quantum Numbers and The H-Atom
- Topic: ionization energy and electron affinty
- Replies: 3
- Views: 179
Re: ionization energy and electron affinty
The difference between electron affinity and ionization energy is that electron affinity gives the amount of energy released when an atom gains an electron, and ionization energy is the amount of energy required to remove an electron from an atom.
- Sat Oct 26, 2019 10:31 pm
- Forum: Quantum Numbers and The H-Atom
- Topic: Quantum number m
- Replies: 3
- Views: 212
Re: Quantum number m
The magnetic quantum number m l determines the number of orbitals and their orientation within a subshell. Consequently, its value depends on the orbital angular momentum quantum number l. Given a certain l, m l is an interval ranging from –l to +l, so it can be zero, a negative integer, or a positi...
- Sat Oct 26, 2019 10:21 pm
- Forum: Quantum Numbers and The H-Atom
- Topic: Quantum Numbers
- Replies: 7
- Views: 258
Re: Quantum Numbers
Electron spin, s, has only two possible values: 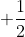 and
and 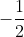 , representing whether the electron is "spin - up" or "spin - down," respectively. Electron spin determines if an atom will or will not generate a magnetic field.
, representing whether the electron is "spin - up" or "spin - down," respectively. Electron spin determines if an atom will or will not generate a magnetic field.
- Sat Oct 26, 2019 10:08 pm
- Forum: Wave Functions and s-, p-, d-, f- Orbitals
- Topic: oribital numbers
- Replies: 7
- Views: 263
Re: oribital numbers
The s subshell has 1 orbital that can hold up to 2 electrons, the p subshell has 3 orbitals that can hold up to 6 electrons, the d subshell has 5 orbitals that hold up to 10 electrons, and the f subshell has 7 orbitals with 14 electrons.
- Sun Oct 20, 2019 2:32 pm
- Forum: Photoelectric Effect
- Topic: When energy is equal to work function
- Replies: 9
- Views: 993
Re: When energy is equal to work function
In the photoelectric effect, if the energy of the photon is equal to that of the work function (i.e. no kinetic energy), what would happen is that the energy threshold will be met and the electron will be ejected.
- Sun Oct 20, 2019 2:29 pm
- Forum: Bohr Frequency Condition, H-Atom , Atomic Spectroscopy
- Topic: Frequencies
- Replies: 7
- Views: 480
Re: Frequencies
In general, no memorization is required to solve a problem - everything you need will be given on the formula sheet. However, in this case, knowing the region of the frequency / wavelength of the electromagnetic spectrum will certainly be beneficial for you, both in terms of your chemistry knowledge...
- Sun Oct 20, 2019 2:16 pm
- Forum: DeBroglie Equation
- Topic: Mass of electron and photon
- Replies: 5
- Views: 309
Re: Mass of electron and photon
In general, no memorization is required. All the information you will need to solve a problem will be given on the formula sheet.
- Sun Oct 20, 2019 2:09 pm
- Forum: Einstein Equation
- Topic: unit of energy
- Replies: 7
- Views: 1030
Re: unit of energy
keV is the abbreviation for kiloelectron volts, a unit of energy equivalent to the kinetic energy gained by an electron falling through a potential of 1 volt.
- Sun Oct 20, 2019 2:07 pm
- Forum: Einstein Equation
- Topic: Units for E [ENDORSED]
- Replies: 4
- Views: 699
Re: Units for E [ENDORSED]
The unit for E is always implied as being J / photons, but if you want to convert to J / atoms, you must use Avogadro's number.
- Sun Oct 13, 2019 10:46 pm
- Forum: Einstein Equation
- Topic: Final Grade
- Replies: 5
- Views: 5934
Re: Final Grade
Our final grade is calculated on a point system (specifically 500 points). Take a peak at the syllabus posted for more information.
- Sun Oct 13, 2019 10:35 pm
- Forum: Trends in The Periodic Table
- Topic: Atomic Radius
- Replies: 16
- Views: 949
Re: Atomic Radius
The effect shielding has on the atomic radius is that it prevents outer electrons from being attracted to the nucleus. Thus, since they are loosely held, the resulting atomic radius is large.
- Sun Oct 13, 2019 10:24 pm
- Forum: Trends in The Periodic Table
- Topic: Element Names
- Replies: 8
- Views: 1031
Re: Element Names
In general, no memorization is required for tests, but you should be familiar with the majority of the elements listed on the periodic table.
- Sun Oct 13, 2019 10:20 pm
- Forum: Trends in The Periodic Table
- Topic: electronegativity
- Replies: 10
- Views: 3328
Re: electronegativity
Electronegativity is a chemical property that describes the tendency of an atom to attract a shared pair of electrons toward itself. Electronegativities generally increase from left to right across a period and generally decrease from top to bottom within a group.
- Sun Oct 13, 2019 10:11 pm
- Forum: Einstein Equation
- Topic: E=hv
- Replies: 5
- Views: 240
Re: E=hv
In this case, the equation says that the energy of a particle of light is proportional to its frequency by a constant factor. Therefore, this equation can be used to calculate the energy required to remove an electron.
- Thu Oct 03, 2019 9:22 am
- Forum: Accuracy, Precision, Mole, Other Definitions
- Topic: Formula Units
- Replies: 6
- Views: 534
Re: Formula Units
Formula units are empirical formulas of any ionic or covalent network solid compound used as an independent entity for stoichiometric calculations. It is the lowest whole number ratio of ions represented in an ionic compound. There are 6.022 * 10 ^ {23} (Avogadro's number) formula units in 1 mole of...
- Thu Oct 03, 2019 9:12 am
- Forum: Molarity, Solutions, Dilutions
- Topic: Molarity
- Replies: 9
- Views: 378
Re: Molarity
Molarity represents the concentration of a solution (specifically the number of moles of solute per liter of solution). You would need to calculate molarity in a wide variety of various situations, including calculating the moles of a solution, calculating the volume of a solution, calculating the m...
- Thu Oct 03, 2019 9:03 am
- Forum: Balancing Chemical Reactions
- Topic: Unequal coefficients
- Replies: 7
- Views: 299
Re: Unequal coefficients
No, we cannot use fractions to balance chemical equations. We can use fractions in the process of balancing chemical equations, but in the end we should obtain whole number coefficients by multiplying each and every coefficient by the denominators of each and every fraction coefficient.
- Thu Oct 03, 2019 8:54 am
- Forum: Balancing Chemical Reactions
- Topic: balancing reaction law
- Replies: 4
- Views: 711
Re: balancing reaction law
In chemical reactions atoms are not created or destroyed. Total mass before = total mass after. The law of conservation of mass states that the total atoms of reactants = the total atoms of products, so you cannot add reactants or products to a chemical equation.
- Thu Oct 03, 2019 8:49 am
- Forum: Empirical & Molecular Formulas
- Topic: Empirical Formula Purpose
- Replies: 13
- Views: 3965
Re: Empirical Formula Purpose
The empirical formula of a compound gives the simplest whole - number ratio between the numbers of atoms of all the elements present in the compound.

