Search found 58 matches
- Sat Dec 07, 2019 5:35 pm
- Forum: Calculating pH or pOH for Strong & Weak Acids & Bases
- Topic: Determining acidity of solution
- Replies: 1
- Views: 190
Re: Determining acidity of solution
In a strong acid-strong base reaction, both the acid and base ionize completely, so the concentration of H3O+ and OH- would be equal assuming that the concentrations of the acid and base are equal. In a strong acid-weak base reaction, there would be H3O+ ions leftover after completion of the reactio...
- Sat Dec 07, 2019 5:08 pm
- Forum: Calculating pH or pOH for Strong & Weak Acids & Bases
- Topic: HClO4 VS. H3PO4
- Replies: 1
- Views: 166
Re: HClO4 VS. H3PO4
Cl is definitely more electronegative than P which does account for why HClO4 is stronger, but you must look at the stability of the anion (conjugate base). ClO-4 has more resonance than H2PO4- (you can look up their structures on google), so it allows for more delocalization of the negative charge,...
- Sat Dec 07, 2019 5:04 pm
- Forum: Lewis Acids & Bases
- Topic: acid base reactions
- Replies: 2
- Views: 215
Re: acid base reactions
Acids/bases are deprotonated/protonated one at a time. So there would be multiple reactions to show the loss of the protons in polyprotic acids. For example phosphoric acid, 3 protons to get to the phosphate ion. http://guweb2.gonzaga.edu/faculty/cronk/biochem/images/phosphate-triprotic-system.gif
- Sat Dec 07, 2019 5:00 pm
- Forum: Acidity & Basicity Constants and The Conjugate Seesaw
- Topic: KA
- Replies: 3
- Views: 296
Re: KA
Ka's are usually given to you, unless the problem is asking you to calculate the Ka from the given concentrations of the products and reacts, but that won't be dealt with until chem 14b.
- Sat Dec 07, 2019 4:58 pm
- Forum: Acidity & Basicity Constants and The Conjugate Seesaw
- Topic: Relative Acidity
- Replies: 2
- Views: 219
Re: Relative Acidity
If the resulting anion, known as the conjugate base, can have its negative charge delocalized, by resonance or inductive effect (electron-withdrawing ability of other electronegative atoms), it is more stable and thus a stronger acid.
- Sun Dec 01, 2019 11:27 pm
- Forum: Properties & Structures of Inorganic & Organic Acids
- Topic: Why sulfuric acid is stronger than phosphoric acid
- Replies: 4
- Views: 3823
Re: Why sulfuric acid is stronger than phosphoric acid
How did you post the images there? I've tried to do the same to post replies but don't know how. When you find an image on google images or wherever, instead of clicking on "Copy," click on "Copy image address. Then you would paste the image address inside after clicking on "Img...
- Sun Dec 01, 2019 10:04 pm
- Forum: Lewis Acids & Bases
- Topic: Relative acidity
- Replies: 4
- Views: 305
Re: Relative acidity
There are no rules in distinguishing strong or weak acids. There are trends/guidelines to help you know/predict if an acid is strong or weak relative to another one. You can look down a group, across a period, and or see if there is resonance and or inductive effect.
- Sun Dec 01, 2019 9:46 pm
- Forum: Lewis Acids & Bases
- Topic: Metal Oxides and Strong Bases
- Replies: 2
- Views: 214
Re: Metal Oxides and Strong Bases
Generally, a strong base, like NaOH, ionizes completely in water. So metal oxides, formed from alkali and alkaline earth metal with oxygen, such as Na2O, would react with water to form a strong base such as NaOH. This is because the oxygen atom acts as a lewis base (donates electron pair) to deproto...
- Sun Dec 01, 2019 9:19 pm
- Forum: Properties & Structures of Inorganic & Organic Acids
- Topic: Why sulfuric acid is stronger than phosphoric acid
- Replies: 4
- Views: 3823
Re: Why sulfuric acid is stronger than phosphoric acid
To understand why acids are stronger or weaker relative to each other, it is important to look at the stabilities of the conjugate bases. In general, the more stable a conjugate base is, the stronger its acid is. Often times, resonance is one of strongest contributors to stability because it can off...
- Sun Dec 01, 2019 12:52 am
- Forum: Bronsted Acids & Bases
- Topic: Bronsted or Lewis Definition?
- Replies: 6
- Views: 410
Re: Bronsted or Lewis Definition?
NaOH, the entire compound, is considered a base (a STRONG base, even). A bronsted base is defined as anything that accepts a proton. But the textbook explicitly states that Na+ is the spectator ion and OH- alone is the Bronsted Base. It warns us to not confuse it with what you said about NaOH being...
- Sat Nov 30, 2019 11:21 pm
- Forum: Calculating pH or pOH for Strong & Weak Acids & Bases
- Topic: strong or weak base?
- Replies: 13
- Views: 911
Re: strong or weak base?
A base in general is a species that can accept a proton to form hydroxide ions. A strong base is one that can readily accept this proton. This means that if you were to have a solution containing a strong base, like NaOH, this species will readily dissociate to form an excess of hydroxide ions. Orga...
- Sat Nov 30, 2019 10:53 pm
- Forum: Bronsted Acids & Bases
- Topic: Bronsted or Lewis Definition?
- Replies: 6
- Views: 410
Re: Bronsted or Lewis Definition?
NaOH, the entire compound, is considered a base (a STRONG base, even). A bronsted base is defined as anything that accepts a proton.
- Sat Nov 23, 2019 4:41 pm
- Forum: Biological Examples
- Topic: Structure of Heme Complex with Sickle Cell Disorder
- Replies: 1
- Views: 130
Structure of Heme Complex with Sickle Cell Disorder
When Dr. Lavelle was discussing the heme complex for myoglobin for biological examples, it made me wonder what the structure of the heme complex would look like for a person with sickle cell trait or sickle cell anemia. I know that the mutation from sickle cell causes the red blood cells to clump up...
- Sat Nov 23, 2019 4:25 pm
- Forum: Biological Examples
- Topic: Example of a Cage like Molecule
- Replies: 2
- Views: 211
Re: Example of a Cage like Molecule
Another example is chlorophyll where Mg+2 binds to 4 nitrogen atoms.
- Sat Nov 23, 2019 4:21 pm
- Forum: Hybridization
- Topic: Hybridization with lone pairs on central atom
- Replies: 6
- Views: 466
Re: Hybridization with lone pairs on central atom
Hybridization is based on the number of regions of electron density.
- Sat Nov 23, 2019 4:15 pm
- Forum: Biological Examples
- Topic: Myoglobin
- Replies: 3
- Views: 216
Re: Myoglobin
The oxygen binds to the Fe+2 ion in the heme complex.
- Sat Nov 23, 2019 4:05 pm
- Forum: Biological Examples
- Topic: Biological Examples
- Replies: 10
- Views: 529
Re: Biological Examples
Cis-platin and how it interacts with the guanine in DNA. I feel like he'd put this because he spent a good amount of time explaining it.
- Sun Nov 17, 2019 9:24 pm
- Forum: Interionic and Intermolecular Forces (Ion-Ion, Ion-Dipole, Dipole-Dipole, Dipole-Induced Dipole, Dispersion/Induced Dipole-Induced Dipole/London Forces, Hydrogen Bonding)
- Topic: bp trend?
- Replies: 2
- Views: 187
Re: bp trend?
Heavier halogens have more electrons in their shell which increases Van Der Waal forces and boiling point Not just heavier halogens, larger molecules, in general, have stronger Van der Waal forces (induced dipole-induced dipole, London dispersion forces) because they have more surface area for dist...
- Sun Nov 17, 2019 9:14 pm
- Forum: Hybridization
- Topic: d orbital
- Replies: 3
- Views: 258
Re: d orbital
It allows expanded octets to form when the central atom is in the third period and down the periodic table. It can accommodate more electrons, as atoms in the p-block of the third, fourth, and fifth period have empty d-orbitals, and the examples of this in VSEPR are trigonal bipyramidal and octahedr...
- Sun Nov 17, 2019 9:05 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Lone vs. Bonded Electrons
- Replies: 3
- Views: 324
Re: Lone vs. Bonded Electrons
Bonding electrons are stabilized in the covalent bonds between the atoms with bond length, strength, order, and hybridization being taken into account. With lone pairs, charge density is more spread out (electron cloud around the atom), so their repulsion strength is stronger than bonded electrons.
- Sun Nov 17, 2019 9:00 pm
- Forum: Bond Lengths & Energies
- Topic: Polarizability
- Replies: 4
- Views: 349
Re: Polarizability
Polarizability is a concept of size with anions and cations. Since anions are larger than their respective neutral states because of having more electrons, it is easier to distort (move the electrons around the atom) the electron cloud. With respect to cations, they have polarizing strength, meaning...
- Sun Nov 17, 2019 8:54 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Bond Angles
- Replies: 7
- Views: 464
Re: Bond Angles
Also, atomic size affects bond angles because a larger atom would have more electrons, thus having more electrons, making the bond angles smaller.
- Sun Nov 10, 2019 11:57 pm
- Forum: Bond Lengths & Energies
- Topic: Boiling Points
- Replies: 2
- Views: 198
Re: Boiling Points
Compounds with higher boiling points generally means that the bonds present in the compound are stronger, since it will require a higher temperature for the substance to change state. It requires a higher temperature because the bonds are stronger and therefore harder to break apart. This could sig...
- Sun Nov 10, 2019 11:53 pm
- Forum: Dipole Moments
- Topic: Dipole dipole forces
- Replies: 2
- Views: 274
Re: Dipole dipole forces
A dipole-dipole force is between two dipoles like HCl and HCl. A dipole-induced dipole is between a dipole and a nonpolar molecule. The dipole distorts the electron cloud of the molecule, so the electrons shifts around the nucleus, causing a partial positive and partial negative charge, that becomes...
- Sun Nov 10, 2019 11:46 pm
- Forum: Coordinate Covalent Bonds
- Topic: carbon monoxide formal charges
- Replies: 5
- Views: 876
Re: carbon monoxide formal charges
Because that is the only way to attain a neutral structure with an overall charge of 0.
- Sun Nov 10, 2019 8:00 pm
- Forum: Bond Lengths & Energies
- Topic: bond lengths
- Replies: 3
- Views: 212
Re: bond lengths
You would know depending on whether it is a single, double, or triple bond. If the types of bonds are the same, then you would know if the bond lengths are shorter or longer based on the periods the atoms are on. Because atomic radii increase from top to bottom on the periodic table, the bond length...
- Sun Nov 10, 2019 7:39 pm
- Forum: Ionic & Covalent Bonds
- Topic: Both types of bonds
- Replies: 6
- Views: 382
Re: Both types of bonds
Yes, but ionic bonds are almost always between the cation and anion whereas covalent bonds are shared between atoms. Ionic bonds can have covalent character while covalent bonds can have ionic character which is why Dr. Lavelle didn't specify if the bond is ionic or covalent when the difference in e...
- Sun Nov 10, 2019 7:34 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: molar mass and attractive interactions
- Replies: 3
- Views: 157
Re: molar mass and attractive interactions
An increase in size and molar mass means that there is more surface area for the atoms/molecules to have London dispersion forces. Although instantaneous and short-lived, the interaction is stronger with larger atoms and molecules because there are more electron distortion and polarizability of of e...
- Sun Nov 10, 2019 5:23 pm
- Forum: Interionic and Intermolecular Forces (Ion-Ion, Ion-Dipole, Dipole-Dipole, Dipole-Induced Dipole, Dispersion/Induced Dipole-Induced Dipole/London Forces, Hydrogen Bonding)
- Topic: Hydrogen bonding
- Replies: 7
- Views: 426
Re: Hydrogen bonding
Hydrogen bonding is a special type of dipole-dipole interaction due to the high electronegativity of Nitrogen, Oxygen, and Fluorine, so hydrogen bonds occur in water, ammonia, and hydrogen fluoride. They can only occur when there are lone pairs around those atoms.
- Sun Nov 10, 2019 5:21 pm
- Forum: Polarisability of Anions, The Polarizing Power of Cations
- Topic: High Distortion
- Replies: 4
- Views: 290
Re: High Distortion
High distortion means that the electron cloud of the electron-rich anion can be easily distorted by a polarizing cation. The reason is that the high number of electrons can be easily moved/shifted around the nucleus as another charged ion or molecule gets close to the vicinity of it.
- Sun Nov 03, 2019 11:29 pm
- Forum: Lewis Structures
- Topic: double bonding with halogens
- Replies: 3
- Views: 154
Re: double bonding with halogens
Chlorine can form double bonds if it is the central atom because it can utilize its 3d orbitals to have an expanded octet. Generally, when bonded to a central atom, halogens usually form single bonds.
- Sun Nov 03, 2019 6:48 pm
- Forum: Ionic & Covalent Bonds
- Topic: Ionic and Covalent Characteristics
- Replies: 3
- Views: 210
Re: Ionic and Covalent Characteristics
The larger the difference between electronegativity is the extent to which a covalent bond has ionic properties because the bond gets more polar as the difference in electronegativity increases.
- Sun Nov 03, 2019 3:54 pm
- Forum: Ionic & Covalent Bonds
- Topic: Distortion
- Replies: 4
- Views: 236
Re: Distortion
Anions are generally much larger than cations because they gain electrons to attain the octet, and we already know that anions are negative ions while cations are positive ions. When a cation gets closer to the vicinity of an anion, the positive charge distorts the electron cloud of the negatively c...
- Sun Nov 03, 2019 2:15 pm
- Forum: Dipole Moments
- Topic: Hydrogen Bond
- Replies: 7
- Views: 415
Re: Hydrogen Bond
A hydrogen bond is a type of dipole-dipole attraction, but it is stronger because the hydrogen atom(s) are covalently bonded to oxygen, nitrogen, or fluorine. Because there are only one proton and one electron in the hydrogen atom, when it is bonded with oxygen, nitrogen, or fluorine, the dipole mom...
- Sun Nov 03, 2019 2:06 pm
- Forum: Dipole Moments
- Topic: Polarity
- Replies: 4
- Views: 169
Re: Polarity
You would know based on the electronegativity of the elements by using the periodic trends and also by formal charges because a negative formal charge would mean the electron density his higher around that atom.
- Sun Nov 03, 2019 2:00 pm
- Forum: Dipole Moments
- Topic: Dipole moments
- Replies: 5
- Views: 322
Re: Dipole moments
A molecule will always be covalent because it is covalently bonded. The dipole moment determines whether the covalent bond has ionic or covalent character because of the charge difference. If there is a dipole moment, it is a polar covalent bond, and as a result of the partial positive and partial n...
- Sun Oct 27, 2019 7:07 pm
- Forum: Formal Charge and Oxidation Numbers
- Topic: Formal Charge and Lone Pairs
- Replies: 4
- Views: 240
Re: Formal Charge and Lone Pairs
You would count the number of electrons in the lone pair and bonds individually. But here's a shortcut to calculate the formal charge…
Formal charge=(#of valence electrons for the atom)-(Total number of lone pair electrons and bonds on the atom)
Formal charge=(#of valence electrons for the atom)-(Total number of lone pair electrons and bonds on the atom)
- Sun Oct 27, 2019 5:54 pm
- Forum: Electron Configurations for Multi-Electron Atoms
- Topic: Effective Nuclear Charge
- Replies: 4
- Views: 251
Re: Effective Nuclear Charge
Yes, a higher number of electrons results in an increase in shielding and a decrease in effective nuclear charge. This is because an increased number of electrons between an atom's positive nucleus and the atom's valence electrons results in both increased attraction between the internal electrons a...
- Sun Oct 27, 2019 2:25 pm
- Forum: Trends in The Periodic Table
- Topic: Electron affinity.
- Replies: 8
- Views: 291
Re: Electron affinity.
Electron affinity is the absolute value of the energy associated with an atom in the gas phase gaining of an electron. All this means is that electron affinity describes the ease at which an atom in the gas phase accepts an electron.
- Sun Oct 27, 2019 2:05 pm
- Forum: Formal Charge and Oxidation Numbers
- Topic: Central atom
- Replies: 8
- Views: 360
Re: Central atom
You have to take into account all of the atoms in the Lewis structure and the overall charge of the molecule because it is more stable when the negative formal charge is on the more electronegative atom and the positive formal charge is on the less electronegative atom.
- Sun Oct 27, 2019 2:01 pm
- Forum: Octet Exceptions
- Topic: 8 valence electrons
- Replies: 3
- Views: 218
Re: 8 valence electrons
Any of the elements in the third principal energy level and higher because they can accommodate more electrons with their d- orbitals. Other common examples are Si and Xe.
- Sun Oct 20, 2019 10:33 pm
- Forum: Quantum Numbers and The H-Atom
- Topic: Quantum Numbers: Size of An Atom
- Replies: 4
- Views: 149
Re: Quantum Numbers: Size of An Atom
The reduced electrostatic attraction due to the distance from the nucleus, electron-electron repulsions, and shielding from the inner energy levels and subshells results in an effective nuclear charge, and the result is an increase in the size of the atom.
- Sun Oct 20, 2019 11:20 am
- Forum: Trends in The Periodic Table
- Topic: Ionic radii
- Replies: 11
- Views: 375
Re: Ionic radii
As you go down a group, the radius increases because the principal quantum number (n) increases, but you have you take more into account. The nuclear charge increases because there are more protons pulling electrons, but the effective nuclear charge is affected because the electron-electron repulsio...
- Sun Oct 20, 2019 11:03 am
- Forum: Quantum Numbers and The H-Atom
- Topic: Angular Momentum Quantum #
- Replies: 3
- Views: 153
Re: Angular Momentum Quantum #
The angular momentum quantum number is used to describe the 'shape' of the orbital whether l= 0 (s), 1 (p), 2 (d), or 3 (f).
- Sun Oct 20, 2019 10:58 am
- Forum: Quantum Numbers and The H-Atom
- Topic: Quantum Numbers
- Replies: 9
- Views: 409
Re: Quantum Numbers
In addition to what Chem_Mod said about angular momentum quantum number and magnetic quantum number, the angular momentum quantum number describes the 'shape' of the orbital while the magnetic quantum number describes the orientation in space of the different orbitals of a subshell.
- Thu Oct 17, 2019 4:55 pm
- Forum: Wave Functions and s-, p-, d-, f- Orbitals
- Topic: Orbitals
- Replies: 2
- Views: 74
Re: Orbitals
It will still be 3 and 5 respectively unless the magnetic quantum number, 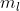 , is given.
, is given.
- Thu Oct 17, 2019 4:43 pm
- Forum: DeBroglie Equation
- Topic: Frequency and wavelength
- Replies: 2
- Views: 117
Re: Frequency and wavelength
You would need to know the energy of the object if you wanted to calculate its frequency using the De Broglie equation because E=hv. You would need to substitute E/v for h into the De Broglie equation to solve for frequency.
- Thu Oct 10, 2019 11:57 pm
- Forum: Properties of Light
- Topic: models of light
- Replies: 5
- Views: 345
Re: models of light
The photon (particle) and wave model are different because, in the photon model, the intensity is proportional to the number of photons present at each instant, while in the wave model, the intensity of electromagnetic radiation is proportional to the square of the amplitude of the wave. These show ...
- Thu Oct 10, 2019 5:25 pm
- Forum: Properties of Electrons
- Topic: Electron energy levels- conceptual stuff
- Replies: 3
- Views: 135
Re: Electron energy levels- conceptual stuff
Electrons can be excited from any principal energy level (n=1, 2, 3, etc). At the same time, when you use Rydberg's equation to calculate the energy change, the final energy level does not have to be where the electron was originally before it was excited. When an electron is excited, it goes back d...
- Thu Oct 10, 2019 5:02 pm
- Forum: Properties of Light
- Topic: Photoelectric Effect: Post-Assessment Question
- Replies: 2
- Views: 95
Re: Photoelectric Effect: Post-Assessment Question
This question is similar to the worked example under the photoelectric effect during the lecture. In part A, you calculated the threshold energy to remove an electron which is the work function, \phi , which is 7.22 x 10^-19 J. Since you are looking for the kinetic energy, E_{k} , you must first cal...
- Thu Oct 10, 2019 4:50 pm
- Forum: Photoelectric Effect
- Topic: Planck's Constant
- Replies: 4
- Views: 170
Planck's Constant
Where does Planck's constant come from? I know that it is used to relate the energy in a photon of electromagnetic radiation to the frequency, but how was it derived? In the field of quantum mechanics, Planck's constant is used literally everywhere, so I'm curious as to where the constant originated...
- Thu Oct 10, 2019 4:33 pm
- Forum: Properties of Electrons
- Topic: Planck's Constant
- Replies: 3
- Views: 183
Re: Planck's Constant
Planck's constant is used to relate the energy in one photon or packet of energy (quanta) of electromagnetic radiation to its frequency. His idea was that a charged particle oscillating at a frequency \nu (nu) can exchange energy with its surroundings by generating or absorbing electromagnetic radia...
- Wed Oct 02, 2019 11:25 pm
- Forum: Limiting Reactant Calculations
- Topic: Methods of identifying Limiting Reactants
- Replies: 5
- Views: 832
Re: Methods of identifying Limiting Reactants
I wouldn't say that this is an official or first choice way of determining the limiting reactant because there are multiple variables that have an influence on this way of conceptualizing the limiting reactant, but from the limiting reagents module, I realized there is a correlation with the stoichi...
- Wed Oct 02, 2019 1:13 pm
- Forum: Balancing Chemical Reactions
- Topic: How do oxidation numbers help you balance equations?
- Replies: 2
- Views: 205
Re: How do oxidation numbers help you balance equations?
I’m not exactly sure what kind of chemical reaction it was when your TA was balancing an equation for one of your discussion problems, but in general, oxidation numbers are essential for oxidation-reduction (redox) reactions. The reason for this is because, in redox reactions, electrons are stripped...
- Wed Oct 02, 2019 12:13 pm
- Forum: Molarity, Solutions, Dilutions
- Topic: Dilution and Molarity Questions
- Replies: 8
- Views: 667
Re: Dilution and Molarity Questions
When doing molarity and dilution problems, it is essential to know which of the given volumes and concentrations correspond with each other. For example, 50mL of a 0.5M sol of A, and you want to calculate the concentration of 250mL of sol B that would neutralize sol A. To solve this, I make sure I k...
- Wed Oct 02, 2019 11:52 am
- Forum: SI Units, Unit Conversions
- Topic: Question about Showing Work
- Replies: 22
- Views: 1074
Re: Question about Showing Work
Would it also be necessary to write out the formula being used in answering a question? I don't think it is necessary to show the formula being used when answering a question because the TA would know which formula you're using when you have the values plugged in already, but it is helpful to have ...
- Wed Oct 02, 2019 9:42 am
- Forum: SI Units, Unit Conversions
- Topic: Question about Showing Work
- Replies: 22
- Views: 1074
Re: Question about Showing Work
In the problems we've done, I would say showing work for conversions such as mL to L wouldn't need to be shown, but for everything else such as molar mass, molarity, and other aspects of dimensional analysis should be shown.
- Wed Oct 02, 2019 9:07 am
- Forum: Balancing Chemical Reactions
- Topic: States of Matter in a Chemical Equation [ENDORSED]
- Replies: 11
- Views: 914
Re: States of Matter in a Chemical Equation [ENDORSED]
If the problem doesn't directly tell you the states of the reactants or products, you can determine it based on the reaction knowing if the reaction occurs in an aqueous solution. Most products from reactions in aqueous solutions are also aqueous. If you know that one of the products is a precipitat...

