Search found 108 matches
- Sat Mar 14, 2020 8:42 pm
- Forum: Arrhenius Equation, Activation Energies, Catalysts
- Topic: Arrhenius equation and activation energies
- Replies: 2
- Views: 286
Re: Arrhenius equation and activation energies
Referring to homework 7D.1 as an example, the problem gives you 2 rate constants at 2 different temperatures and asks you to find the activation energy. In this case, you would rearrange the Arrhenius equation as a difference of T1 and T2 to get ln\frac{k_{2}}{k_{1}} = \frac{E_{a}}{R}(\frac{1}{T...
- Sat Mar 14, 2020 8:32 pm
- Forum: General Rate Laws
- Topic: Steps
- Replies: 2
- Views: 294
Re: Steps
Usually, the problem should tell you whether the elementary step is slow or fast. So, the problem will tell you that there are elementary steps, and then to find the overall reaction you would add the steps together. The slow step is the rate-determining step, so you would use its rate law.
- Sat Mar 14, 2020 8:29 pm
- Forum: General Rate Laws
- Topic: How to use general rate laws to find rates of specific equations?
- Replies: 4
- Views: 542
Re: How to use general rate laws to find rates of specific equations?
Since these are elementary reactions, you can write the rate law using the coefficients. For part a, since there are 2 NO molecules on the reactant side, the rate = k[NO]^2, and it is bimolecular. For part b, there is only 1 Cl2 molecule, so the rate = k[Cl2], and it is unimolecular.
- Sat Mar 14, 2020 8:20 pm
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: R and Nernst equation
- Replies: 2
- Views: 423
Re: R and Nernst equation
The Nernst equation is E = E(standard) - (RT/nF)lnQ. Since R = 8.314 J * K^-1 * mol^-1, it will cancel out with the units for T, temperature, at Kelvin. the mol in R will also cancel with the mol for Faraday's constant, which is C/mol. Then, what you end with for the units of (RT/nF) is J/C, which e...
- Sat Mar 14, 2020 8:15 pm
- Forum: Reaction Mechanisms, Reaction Profiles
- Topic: Units
- Replies: 3
- Views: 335
Re: Units
The units for k will depend on the overall order of the reaction. k has the units of L^2 * mol^-2 * s^-1 when the overall order is 3.
- Sun Mar 08, 2020 7:21 pm
- Forum: Reaction Mechanisms, Reaction Profiles
- Topic: Example 7C.1
- Replies: 1
- Views: 175
Re: Example 7C.1
I think the rate of decomposition relates to the rate of formation in that they are conveyed by the same rate law. For part a, the reaction is NO + NO --> N2O2. The rate law is rate = k[NO]^2. For the decomposition of NO, the rate law is k[NO]^2, and for the rate of formation of N2O2, the rate law i...
- Sun Mar 08, 2020 6:56 pm
- Forum: Reaction Mechanisms, Reaction Profiles
- Topic: 7.23b
- Replies: 1
- Views: 175
7.23b
ClO- + H2O \rightleftharpoons HClO + OH- (fast equilibrium) HClO + I- --> HIO + Cl- (very slow) HIO + OH- \rightleftharpoons +H2O (fast equilibrium) (b) Write the rate law based on this mechanism. Since step 2 is slow, the rate law is rate = k2[HClO][I-]. Then, the solutions manual shows that you sh...
- Sun Mar 08, 2020 6:22 pm
- Forum: Reaction Mechanisms, Reaction Profiles
- Topic: 7.11
- Replies: 1
- Views: 186
7.11
The rate law of the reaction 2NO(g) + 2H2(g) --> N2(g) + 2H2O(g) is rate = k[NO]^2[H2], and the mechanism that has been proposed is Step 1: NO + NO --> N2O2 Step 2: N2O2 + H2 --> N2O + H2O Step 3: N2O + H2 --> N2 + H2O (a) Which step in the mechanism is likely to be rate determining? Explain your an...
- Sun Mar 08, 2020 5:51 pm
- Forum: Arrhenius Equation, Activation Energies, Catalysts
- Topic: 7E.5
- Replies: 1
- Views: 178
7E.5
The hydrolysis of an organic nitrile, a compound containing a –CN group, in basic solution, is proposed to proceed by the following mechanism. Write a complete balanced equation for the overall reaction, list any intermediates, and identify the catalyst in this reaction. Step 1: RCN + OH- --> RCNOH ...
- Sun Mar 08, 2020 5:33 pm
- Forum: Arrhenius Equation, Activation Energies, Catalysts
- Topic: 7E.3A
- Replies: 1
- Views: 230
7E.3A
The presence of a catalyst provides a reaction pathway in which the activation energy of a certain reaction is reduced from 125 kJ/mol to 75 kJ/mol. (a) By what factor does the rate of the reaction increase at 298 K, all other factors being equal? (b) By what factor would the rate change if the reac...
- Mon Mar 02, 2020 5:06 pm
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: Units for lnQ
- Replies: 2
- Views: 290
Units for lnQ
For some problems where you have to calculate the cell potential using the Nernst equation, the solutions manual substitutes concentrations for aqueous species and partial pressures for gases into Q for lnQ. Don't you have to convert the units so that the species in Q are uniformly either in M or at...
- Sun Mar 01, 2020 2:31 am
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: 6L.9
- Replies: 2
- Views: 223
6L.9
(a) Write balanced half-reactions for the redox reaction of an acidified solution of potassium permanganate and iron(II) chloride. (b) Write the balanced equation for the cell reaction and devise a galvanic cell to study the reaction (write its cell diagram). The half reactions are: anode: MnO_{4}^{...
- Sat Feb 29, 2020 6:38 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: 5G. 13
- Replies: 2
- Views: 252
5G. 13
(a) Calculate the reaction Gibbs free energy of I2(g) -->2I(g) at 1200. K (K = 6.8) when the partial pressures of I2 and I are 0.13 bar and 0.98 bar, respectively. (b) Indicate whether this reaction mixture is likely to form reactants, is likely to form products, or is at equilibrium. In the solutio...
- Tue Feb 25, 2020 8:30 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: 6M.11D
- Replies: 1
- Views: 188
6M.11D
Suppose that each of the following pairs of redox couples is combined to form a galvanic cell that generates a current under standard conditions. Identify the oxidizing agent and the reducing agent, write a cell diagram, and calculate the standard cell potential from the standard potentials of the e...
- Mon Feb 24, 2020 4:25 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: 6M.1
- Replies: 4
- Views: 415
6M.1
A student was given a standard Cu(s)|Cu2+(aq) half-cell and another half-cell containing an unknown metal M in 1.00M M(NO3)2(aq) and formed the cell M(s)|M+(aq)||Cu2+(aq)|Cu(s). The cell potential was found to be -0.689 V. What is the value of E(M2+/M)? In this problem, since Cu2+(aq)/Cu(s) is writ...
- Mon Feb 24, 2020 2:21 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: 6L.5B
- Replies: 1
- Views: 192
6L.5B
For the cell diagram for the reaction +I^{-}(aq)\rightarrow I_{2}(s)+Ce^{3+}(aq)) , how do you know that Platinum is included in the cell diagram on both the anode and cathode sides of the diagram?
, how do you know that Platinum is included in the cell diagram on both the anode and cathode sides of the diagram?
- Mon Feb 24, 2020 2:37 am
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: 6L.3D
- Replies: 1
- Views: 225
6L.3D
Write the half-reactions and the balanced equation for the cell reaction for each of the following galvanic cells: (d) Pt(s) | O2 (g) | H+ (aq) || OH- (aq) | O2 (g) | Pt (s) For the oxidation half reaction for the anode, how do you know that O2 (g) and H+ (g) are the reactants and that H2O is the pr...
- Mon Feb 24, 2020 1:10 am
- Forum: Balancing Redox Reactions
- Topic: Oxidation Number of O2 and O3
- Replies: 3
- Views: 467
Oxidation Number of O2 and O3
How do you know that O3 is being reduced in the reaction of O3(aq) --> O2(g) when both O3 and O2 have an oxidation state of 0 since they're both molecules?
- Sun Feb 23, 2020 7:42 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Writing the Cell Reaction from the Cell Diagram
- Replies: 1
- Views: 182
Writing the Cell Reaction from the Cell Diagram
How would you write the reaction equation from the given cell diagram? For example, how would you write the reaction for
Zn(s)|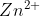 (aq)||
(aq)||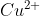 (aq)|Cu(s)?
(aq)|Cu(s)?
Zn(s)|
- Sun Feb 23, 2020 7:37 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Electrochemical cells
- Replies: 2
- Views: 222
Re: Electrochemical cells
A galvanic cell is an electrochemical cell that generates an electrical current from a spontaneous reaction. In other words, if the free energy (delta G) of the reaction is negative, then the reaction proceeds in the forward direction.
- Sun Feb 23, 2020 7:28 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Galvanic Cells
- Replies: 3
- Views: 308
Re: Galvanic Cells
When you're relating free energy to the cell potential, as given by \Delta G=-nFE_{cell} , the cell potential (E) comes from the galvanic cell. So, you'd need to calculate the cell potential from the galvanic cell and its two electrodes – the anode and the cathode. Using the two electrodes, you can ...
- Sun Feb 23, 2020 1:51 am
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: Textbook Example 6N.1
- Replies: 1
- Views: 206
Textbook Example 6N.1
Calculate the equilibrium constant at 25.00 degreesC for the reaction AgCl(s)\rightarrow Ag^{+}(aq)+Cl^{-}(aq) . The equilibrium constant for this reaction is actually the solubility product, Ksp = [Ag+][Cl-], for silver chloride. The two reduction half reactions are R: AgCl&...
- Sun Feb 23, 2020 1:16 am
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Textbook Example 6L.2
- Replies: 1
- Views: 163
Textbook Example 6L.2
Write the reaction for the cell Pt(s)|H2(g)|HCl(aq)|Hg2Cl2(s)|Hg(l). The textbook example shows that the reduction half reaction is Hg_{2}Cl_{2}(s)+2e^{-}\rightarrow 2Hg(l)+2Cl^{-}(aq) and that the oxidation half reaction is H_{2}(g)\rightarrow 2H^{+}(aq)+2e^{...
- Sun Feb 16, 2020 11:18 pm
- Forum: Van't Hoff Equation
- Topic: Assumptions
- Replies: 3
- Views: 279
Re: Assumptions
You have multiple equations for finding the change in entropy. One is \Delta S=\frac{q}{T} . You use this equation when the temperature is constant. The other equation is \Delta S=nCln\frac{T_{2}}{T_{1}} . This equation is used when you have a final temperature that is different from the initial tem...
- Sun Feb 16, 2020 11:05 pm
- Forum: Van't Hoff Equation
- Topic: Significance of Van't Hoff Equation
- Replies: 8
- Views: 608
Significance of Van't Hoff Equation
What is the significance of the Van't Hoff equation? It connects thermodynamics with the equilibrium constant, but is it supposed to tell us anything about a reaction?
- Mon Feb 10, 2020 3:24 am
- Forum: Entropy Changes Due to Changes in Volume and Temperature
- Topic: 4F.17
- Replies: 1
- Views: 180
4F.17
Calculate the standard entropy of vaporization of water at 85 degreesC, given that its standard entropy of vaporization at 100. degreesC is 109.0 J/K*mol and the molar heat capacities at constant pressure of liquid water and water vapor are 75.3 J/K*mol and 33.6 J/K*mol, respectively, in this range....
- Mon Feb 10, 2020 1:55 am
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: 4.17E
- Replies: 1
- Views: 118
4.17E
A technician carries out the reaction 2SO_{2}(g) + O_{2}(g) \rightarrow 2SO_{3}(g) at 25^{o}C and 1.00 atm in a cylinder fitted with a piston and maintained at constant pressure. Initially, 0.030 mol SO_{2} and 0.030 mol O_{2} are present in the cylinder. The technician then ...
- Mon Feb 10, 2020 1:39 am
- Forum: Phase Changes & Related Calculations
- Topic: 4.5
- Replies: 1
- Views: 111
4.5
In 1750, Joseph Black performed an experiment that eventually led to the discovery of enthalpies of fusion. He placed two samples of water, each of mass 150. g, at 0.00 degreesC (one ice and one liquid) in a room kept at a constant temperature of 5.00 degreesC. He then observed how long it took for ...
- Sun Feb 09, 2020 2:24 am
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Calculating Bond Enthalpies
- Replies: 5
- Views: 201
Calculating Bond Enthalpies
Is there a way to figure out the bonds that are being formed and broken without having to draw the structure of the molecule? For example, in the reaction of CH_{3}CHCH_{2}(g) + H_{2}O(g) \rightarrow CH_{3}CH(OH)CH_{3}(g) , would there be a way to figure out the bonds...
- Sun Feb 09, 2020 2:19 am
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Units for Reaction Enthalpy
- Replies: 1
- Views: 94
Units for Reaction Enthalpy
In the textbook solutions, for some answers 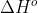 has the units of kJ and for other answers it has the units of kJ/mol. Are kJ and kJ/mol interchangeable as the units for the standard reaction enthalpy?
has the units of kJ and for other answers it has the units of kJ/mol. Are kJ and kJ/mol interchangeable as the units for the standard reaction enthalpy?
- Sun Feb 09, 2020 12:22 am
- Forum: Phase Changes & Related Calculations
- Topic: 4C.13
- Replies: 1
- Views: 193
4C.13
An ice cube of mass 50.0 g at 0.0 degreesC is added to a glass containing 400.0 g of water at 45.0 degreesC. What is the final temperature of the system (see Tables 4A.2 and 4C.1)? Assume that no heat is lost to the surroundings. I know that you use the equation q(ice) = -q(water) since the heat los...
- Sun Feb 02, 2020 9:34 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: 4C.9A
- Replies: 2
- Views: 100
4C.9A
(a) Calculate the heat that must be supplied to a copper kettle of mass 500.0 g containing 400.0 g of water to raise its temperature from 22.0 degrees C to the boiling point of water, 100.0 degrees C. Why do you need to find the heat (q) for copper and the heat (q) for water and add them together? W...
- Sun Feb 02, 2020 9:26 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: 4C.3B
- Replies: 1
- Views: 115
4C.3B
Calculate the final temperature and the change in enthalpy when 765 J of energy is transferred as heat to 0.820 mol Kr(g) at 298 K and 1.00 atm (a) at constant pressure; (b) at constant volume. Treat the gas as ideal. For part b, \Delta U = q, which is 765 J. Then, you use \Delta U to find \Delta H,...
- Sun Feb 02, 2020 9:06 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: 4B.7
- Replies: 2
- Views: 86
4B.7
In a combustion chamber, the total internal energy change produced from the burning of a fuel is -2573 kJ. The cooling system that surrounds the chamber absorbs 947 kJ as heat. How much work can be done by the fuel in the chamber? In the solutions manual, it says that q = –947 kJ. How is q negative ...
- Sun Feb 02, 2020 9:02 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: 4B.5
- Replies: 2
- Views: 195
4B.5
An ideal gas in a cylinder was placed in a heater and gained 5.50 kJ of energy as heat. If the cylinder increased in volume from 345 mL to 1846 mL against an atmospheric pressure of 750. Torr during this process, what is the change in internal energy of the gas in the cylinder? With the equation \De...
- Sun Feb 02, 2020 8:56 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: 4A.13
- Replies: 2
- Views: 111
4A.13
A constant-volume calorimeter was calibrated by carrying out a reaction known to release 3.50 kJ of heat in 0.200 L of solution in the calorimeter (q = -3.50 kJ), resulting in a temperature rise of 7.32 degrees C. In a subsequent experiment, 100.0 mL of 0.200 M HBr(aq) and 100.0 mL of 0.200 M KOH(aq...
- Sun Feb 02, 2020 8:07 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: 4A.9
- Replies: 2
- Views: 105
4A.9
\Delta A piece of copper of mass 20.0 g at 100.0 C is placed in a vessel of negligible heat capacity but containing 50.7 g of water at 22.0 C. Calculate the final temperature of the water. Assume that no energy is lost to the surroundings. In the solutions manual, the equation to solve for the fina...
- Sun Jan 26, 2020 5:54 pm
- Forum: Phase Changes & Related Calculations
- Topic: Why is enthalpy additive?
- Replies: 3
- Views: 151
Re: Why is enthalpy additive?
Since enthalpy is a state property, only its present state is taken into account. Thus, the paths taken to reach that present state do not matter, since only the current state matters. However, the intermediary states are still taken into account, for example with phase changes, or with the bond ent...
- Sun Jan 26, 2020 5:50 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Bond Enthalpies
- Replies: 6
- Views: 151
Re: Bond Enthalpies
The Lewis structures were drawn to just give a better idea of the bonds that would be broken and formed in the reaction. However, we won't have to draw the Lewis structures, but they do give a better idea of the structures and bonds of each molecules so that we know the bond enthalpies for each bond.
- Sun Jan 26, 2020 5:33 pm
- Forum: Phase Changes & Related Calculations
- Topic: Work and State Properties
- Replies: 2
- Views: 145
Work and State Properties
Why is work not a state property when work is force times distance? Since the path doesn't matter for a state property, why would work not be considered a state property?
- Sun Jan 26, 2020 5:30 pm
- Forum: Phase Changes & Related Calculations
- Topic: Steam Burns
- Replies: 9
- Views: 666
Re: Steam Burns
Steam burns are more severe due to the enthalpy of vaporization. At 100 degrees Celsius, water changes from a liquid to gas, and additional energy is needed for the change. However, despite the additional energy, the temperature is the same at 100 degrees Celsius, so both liquid and gaseous water ca...
- Sun Jan 26, 2020 5:26 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Hess's Law
- Replies: 4
- Views: 228
Re: Hess's Law
The method using bond enthalpies is the least accurate since for many different molecules – that are not diatomic– the bond enthalpies are averages.
- Mon Jan 20, 2020 12:03 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: 6E.1
- Replies: 1
- Views: 83
6E.1
Calculate the pH of 0.15 M H2SO4(aq) at 25 degrees C. For polyprotic acids, I thought you only needed to calculate the first Ka value and consider the other deprotonations as insignificant. However, the solutions manual shows that the first ionization is complete but the second one isn't, so you cal...
- Sun Jan 19, 2020 5:11 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: pkB and pkA
- Replies: 1
- Views: 45
Re: pkB and pkA
The lower the pKa or pKb, the stronger is the acid or the base.
- Sun Jan 19, 2020 5:10 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: conjugate seesaw
- Replies: 4
- Views: 137
Re: conjugate seesaw
[H3O+][OH-] = 10^-14 For this equation if the concentration of H3O+ is large, then the concentration of OH- must be low since their product is always a constant at 10^-14, and vice versa. pKa + pKb = pKw Similarly, if pKa is large, then pKb is low since their sum always equals pKw, which is 14, and ...
- Sun Jan 19, 2020 2:52 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: 6D.3a
- Replies: 1
- Views: 97
6D.3a
(a) When the pH of 0.10 M HClO2(aq) was measured, it was found to be 1.2. What are the values of Ka and pKa of chlorous acid? To find [H3O+], you take the antilog of the pH, and [H3O+] also equals [ClO2-]. For the Ka equation, Ka = [H3O+][ClO2-]/[HClO2]. How do you know that [HClO2] = (.10 - .06) wh...
- Sun Jan 19, 2020 2:29 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: 6B.11B
- Replies: 2
- Views: 128
6B.11B
A student added solid Na2O to a volumetric flask of volume 200.0 mL, which was then filled with water, resulting in 200.0mL of NaOH solution. Then 5.00 mL of the solution was transferred to another volumetric flask and diluted to 500.0 mL. The pH of the diluted solution is 13.25. (b) What mass of Na...
- Sun Jan 19, 2020 2:23 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: 6A.21
- Replies: 1
- Views: 67
6A.21
The value of Kw for water at body temperature (37 C) is 2.1 x 10^-14. (a) What is the molar concentration of H3O+ ions at 37 degrees C? (b) What is the molar concentration of OH- in neutral water at 37 degrees C? For part a, the solutions manual shows that you use the expression Kw=[H3O+][OH-] and s...
- Sun Jan 12, 2020 3:32 pm
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Changes in pressure
- Replies: 4
- Views: 261
Changes in pressure
I know that the quick way to explain the changes in pressure is that if the volume decreases and there are more moles of gas on the left, then the reaction proceeds to the right and vice versa. But why is this the short way? If you know that the volume decreases, then you also know that there is sti...
- Sun Jan 12, 2020 3:25 pm
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: 5J.13
- Replies: 4
- Views: 128
Re: 5J.13
Since the K value at 700K is smaller, it means that more reactants are formed since K = Products/Reactants. Since there are more reactants, it means that at 700K, there will be less products and so less ammonia will be present at the higher temperature of 700K.
- Sun Jan 12, 2020 3:23 pm
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: 5.61
- Replies: 3
- Views: 169
5.61
The overall photosynthesis reaction is 6CO2(g) + 6H2O(l) \rightarrow C6H12O6(aq) + 6O2(g), and \Delta H = +2802 kJ. Suppose that the reaction is at equilibrium. State the effect that each of the following changes will have on the equilibrium composition: tends to shift toward the formation of reacta...
- Wed Jan 08, 2020 9:40 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: 5I.13
- Replies: 2
- Views: 123
Re: 5I.13
.001 M is the initial concentration of F2 before equilibrium is reached, but at equilibrium, the concentration of F2 is 8.3 x 10^-4M. Cl2 is more stable because its K value, the equilibrium constant, is smaller. This means that at equilibrium there are more reactants than products, so there is more ...
- Wed Jan 08, 2020 9:31 pm
- Forum: Non-Equilibrium Conditions & The Reaction Quotient
- Topic: 5I.9
- Replies: 3
- Views: 207
5I.9
For the reaction H2(g) + I2(g) \rightleftharpoons 2 HI(g), K=160. at 500. K. An analysis of a reaction mixture at 500. K showed that it had the composition PH2 = 0.20 bar, PI2 = 0.10 bar, and PHI = 0.10 bar. (a) Calculate the reaction quotient. (b) Is the reaction mixture at equilibrium? (c) If not,...
- Sat Dec 07, 2019 9:54 pm
- Forum: Trends in The Periodic Table
- Topic: Mg2+ and F-
- Replies: 1
- Views: 244
Mg2+ and F-
How does Mg2+ have a larger radius than F- when F- has a greater effective nuclear charge since it is further to the right on the periodic table and when Mg2+ is in a higher energy shell?
- Thu Dec 05, 2019 3:18 am
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: EDTA
- Replies: 3
- Views: 292
EDTA
Why don't you include the doubly bonded oxygen atoms when counting the sites where EDTA can bind to a transition metal? So, instead of 6 attachment sites, why can't there be 10, with the 4 included double bonded oxygen atoms?
- Thu Dec 05, 2019 2:48 am
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: 9C.5
- Replies: 1
- Views: 139
9C.5
Which of the following ligands can be polydentate? If the ligand can be polydentate, give the maximum number of places on the ligand that can bind simultaneously to a single metal center: (b) (CO3)2- (d) oxalate For (CO3)2-, all the 3 oxygen atoms have lone pairs, so why is the maximum number for th...
9C.3D
(d) Write the formula for: sodium bisoxalato(diaqua)ferrate(III)
Why is the formula Na[Fe(OH2)2(C2O4)2] instead of Na[Fe(C2O4)2(OH2)2]? If the ligands are written in alphabetical order, why does (OH2)2 go in front of (C2O4)2 in the formula?
Why is the formula Na[Fe(OH2)2(C2O4)2] instead of Na[Fe(C2O4)2(OH2)2]? If the ligands are written in alphabetical order, why does (OH2)2 go in front of (C2O4)2 in the formula?
- Thu Dec 05, 2019 2:28 am
- Forum: Naming
- Topic: Naming Versions
- Replies: 2
- Views: 116
Naming Versions
Can we use the new IUPAC name conventions for naming coordination compounds as used in the examples in the textbook, or do we have to go with the version given on the chart on Dr. Lavelle's website?
- Wed Dec 04, 2019 2:26 am
- Forum: Interionic and Intermolecular Forces (Ion-Ion, Ion-Dipole, Dipole-Dipole, Dipole-Induced Dipole, Dispersion/Induced Dipole-Induced Dipole/London Forces, Hydrogen Bonding)
- Topic: Melting point of solid argon vs. xenon
- Replies: 2
- Views: 753
Melting point of solid argon vs. xenon
Why is the melting point of xenon higher than that of argon?
- Sun Dec 01, 2019 4:52 pm
- Forum: Lewis Acids & Bases
- Topic: Focus 6 #13
- Replies: 1
- Views: 246
Focus 6 #13
Draw the Lewis structure of boric acid, B(OH)3. (a) Is resonance important for its description? (b) The proton transfer equilibrium for boric acid is given in a footnote to Table 6C.1. In that reaction does boric acid act as a Lewis acid, a Lewis base, or neither? Justify your answer by using Lewis ...
- Sun Dec 01, 2019 4:44 pm
- Forum: Bronsted Acids & Bases
- Topic: Distinguishing between the different definitions of acids and bases
- Replies: 2
- Views: 173
Distinguishing between the different definitions of acids and bases
What are the differences between the Bronsted, Lewis, and Arrhenius definitions of acids and bases? How does each definition make identifying acids and bases different?
- Thu Nov 28, 2019 1:44 am
- Forum: Conjugate Acids & Bases
- Topic: 6A.3: Differentiating between acids and bases
- Replies: 2
- Views: 249
6A.3: Differentiating between acids and bases
How do you know if a molecule accepts H+ from H2O or donates H+ to H2O without looking at a table? For example, the question asks to Write the chemical equations for the proton transfer equilibria of the following acids in aqueous solution and identify the conjugate acid–base pairs in each case: (d)...
- Tue Nov 26, 2019 6:55 pm
- Forum: Identifying Acidic & Basic Salts
- Topic: J.9B
- Replies: 1
- Views: 237
J.9B
Identify the salt that is produced from the acid–base neutralization reaction between (b) ammonia and phosphoric acid. I know that phosphoric acid is a triprotic acid, from its formula H3PO4. The first equation for the reaction between ammonia and phosphoric acid is: NH3 + H3PO4 -> NH4H2PO4. How do ...
- Mon Nov 25, 2019 11:32 pm
- Forum: Bronsted Acids & Bases
- Topic: J.1
- Replies: 3
- Views: 253
J.1
Identify each compound as either a Brønsted acid or a Brønsted base: (a) NH3 ; (b) HBr; (c) KOH; (d) H2SO3 ; (e) Ca(OH)2 .
Is there a strategy for identifying each compound as a Bronsted acid/base?
Is there a strategy for identifying each compound as a Bronsted acid/base?
- Mon Nov 25, 2019 8:14 pm
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: 9C.5
- Replies: 1
- Views: 136
9C.5
Which of the following ligands can be polydentate? If the ligand can be polydentate, give the maximum number of places on the ligand that can bind simultaneously to a single metal center: (a) HN(CH2CH2NH2)2; (b) (CO3)2-; (c) H2O; (d) oxalate. How do you determine if the ligands are polydentate? And ...
- Tue Nov 19, 2019 8:11 pm
- Forum: Hybridization
- Topic: Ring Structure
- Replies: 2
- Views: 207
Ring Structure
When a molecule is drawn as a ring structure, are there still lone pairs on the atoms if they don't form octets and even if we can't see it? And we'd still include them in the hybridization?
- Tue Nov 19, 2019 11:59 am
- Forum: Interionic and Intermolecular Forces (Ion-Ion, Ion-Dipole, Dipole-Dipole, Dipole-Induced Dipole, Dispersion/Induced Dipole-Induced Dipole/London Forces, Hydrogen Bonding)
- Topic: H2SeO4
- Replies: 2
- Views: 163
H2SeO4
How does H2SeO4 have a dipole-dipole moment when the oxygen atoms are arranged around Se, with 2 H atoms attached to 2 of the O atoms? Don't the dipole moments cancel out for each oxygen?
- Tue Nov 19, 2019 11:39 am
- Forum: Dipole Moments
- Topic: NH2OH
- Replies: 2
- Views: 302
NH2OH
How is NH2OH polar?
- Tue Nov 19, 2019 1:23 am
- Forum: Interionic and Intermolecular Forces (Ion-Ion, Ion-Dipole, Dipole-Dipole, Dipole-Induced Dipole, Dispersion/Induced Dipole-Induced Dipole/London Forces, Hydrogen Bonding)
- Topic: States and Intermolecular forces
- Replies: 4
- Views: 300
States and Intermolecular forces
How do the states of the molecules (solid, liquid, gas) relate to intermolecular forces? Do solids have stronger intermolecular forces?
- Tue Nov 19, 2019 1:22 am
- Forum: Interionic and Intermolecular Forces (Ion-Ion, Ion-Dipole, Dipole-Dipole, Dipole-Induced Dipole, Dispersion/Induced Dipole-Induced Dipole/London Forces, Hydrogen Bonding)
- Topic: Strength of Intermolecular Forces
- Replies: 3
- Views: 305
Strength of Intermolecular Forces
Explain the trend in the boiling points of the hydrogen halides: HCl, -85 degrees Celsius; HBr, -67 degrees Celsius; HI, -5 degrees celsius. How would the boiling points depend on the forces of these molecules?
- Mon Nov 18, 2019 2:54 am
- Forum: Hybridization
- Topic: 2F.15
- Replies: 2
- Views: 98
2F.15
Noting that the bond angle of an sp3 hybridized atom is 109.5 degrees and that of an sp2 hybridized atom is 120 degrees, do you expect the bond angle between two hybrid orbitals to increase or decrease as the s-character of the hybrids is increased? Can someone explain this to me? I don't understand...
- Sun Nov 17, 2019 11:25 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: 2E.27A
- Replies: 1
- Views: 89
2E.27A
For part A, if you weren't given the information that C5H5N, pyridine, has a similar structure to benzene, how would you know to draw it as a ring structure?
- Sun Nov 17, 2019 6:34 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: 2E.25
- Replies: 3
- Views: 113
2E.25
How are CH2Cl2 and SF4 polar when their dipole moments cancel in their Lewis structures?
- Sun Nov 17, 2019 5:48 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: 2E.19A
- Replies: 2
- Views: 158
2E.19A
When drawing the Lewis structure for (S2O3)2-, does it matter which Lewis structure I draw? One structure consists of the central S atom with 3 double bonds attached to O and one single bond attached to S. The other structure is 2 double bonds between the central S atom and 2 O atoms, and 1 single b...
- Sun Nov 17, 2019 5:28 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: 2E.15B
- Replies: 1
- Views: 140
2E.15B
For part b, the book solutions manual states that TeCl4 has a seesaw molecular shape, but its bond angles are 90 degrees and 120 degrees. If Te has a lone pair on an equatorial position in its seesaw structure, why do the bond angles equal 90 degrees and 120 degrees? Shouldn't they be slightly less ...
- Sun Nov 17, 2019 5:14 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: 2E.13
- Replies: 1
- Views: 118
2E.13
For I3-, the shape is linear with 3 lone pairs around the central I atom. Why does the bond angle equal 180 degrees instead of being slightly below 180 degrees when there are 3 lone pairs of electrons around the central I atom? Wouldn't those lone pairs slightly push down on the bond angles?
- Sun Nov 17, 2019 4:14 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: XeF2
- Replies: 5
- Views: 379
XeF2
How is XeF2 polar when it has 3 lone pairs? How do the dipole moments cancel when there are two lone pairs above Xe and one lone pair below Xe?
- Sun Nov 10, 2019 4:39 pm
- Forum: Lewis Structures
- Topic: More than 8 electrons in structure?
- Replies: 4
- Views: 181
Re: More than 8 electrons in structure?
Yes, the d-subshell and f-subshell allows electrons to occupy more orbitals, therefore expanding its octet.
- Sun Nov 10, 2019 4:37 pm
- Forum: Trends in The Periodic Table
- Topic: Electronegativity
- Replies: 9
- Views: 513
Re: Electronegativity
Electronegativity is the ability of an atom to attract shared electrons toward itself. Electronegativity generally decreases down a group because there is more distance between the nucleus and the outer valence shell and increases across a period because the charge on the nucleus increases due to th...
- Sun Nov 10, 2019 4:27 pm
- Forum: Heisenberg Indeterminacy (Uncertainty) Equation
- Topic: Using the equation
- Replies: 2
- Views: 220
Re: Using the equation
Can somebody please explain to me how we would use this equation? The example in the textbook makes sense but when I go to actually apply it it does not make sense at all. Like they'll give us the weight in grams but I'm not sure how that's relevant. Thank you! The equation is (delta p)(delta x) >/=...
- Sun Nov 10, 2019 4:22 pm
- Forum: Dipole Moments
- Topic: Polarity
- Replies: 8
- Views: 249
Polarity
How do you know if a molecule is polar? Do you always have to draw it out to see if it's polar or not?
- Mon Nov 04, 2019 4:47 am
- Forum: Ionic & Covalent Bonds
- Topic: Valence Electrons and Electron Configuration
- Replies: 1
- Views: 181
Valence Electrons and Electron Configuration
If you're forming an ion from an atom that has its d-orbitals filled, then are the d-orbitals still considered valence electrons? For example, the electron configuration of Ga is [Ar] 3d^10 4s^2 4p^1. But if you want to form the Ga ion, you would only remove the electrons in the 4s and 4p orbitals t...
- Sun Nov 03, 2019 1:38 am
- Forum: Octet Exceptions
- Topic: Tungsten Electronic Configuration [ENDORSED]
- Replies: 2
- Views: 95
Tungsten Electronic Configuration [ENDORSED]
Why is the electronic configuration for tungsten [Xe]4f^14 5d^4 6s^2? I thought that the d-subshell wants to have half-filled orbitals, so that the electronic configuration would be [Xe]4f^14 5d^5 6s^1.
- Sun Nov 03, 2019 1:25 am
- Forum: Resonance Structures
- Topic: Defining Resonance Structures
- Replies: 3
- Views: 156
Defining Resonance Structures
Are resonance structures only the Lewis structures by which you can move the double triple bonds? Or do they include all the structures that you can draw for a molecule? For example, with N2O, you can draw different structures with single, double, or triple bonds, so would all those structures be co...
- Tue Oct 29, 2019 8:53 pm
- Forum: Electronegativity
- Topic: 3F. 5C
- Replies: 1
- Views: 201
3F. 5C
Suggest, giving reasons, which substance in each of the following pairs is likely to have the higher normal melting point
(Lewis structures may help your arguments):
(c) CHI3 or CHF3.
How does CHI3 have a higher melting point than CHF3 when CHF3 is more polar due to the F atom?
(Lewis structures may help your arguments):
(c) CHI3 or CHF3.
How does CHI3 have a higher melting point than CHF3 when CHF3 is more polar due to the F atom?
- Mon Oct 28, 2019 9:49 pm
- Forum: Lewis Structures
- Topic: Drawing Lewis Structures
- Replies: 4
- Views: 210
Drawing Lewis Structures
When drawing Lewis structures, are you supposed to always draw the different resonance structures and find the formal charges? For example, for 2B. 11C, when drawing H2C(NH2)COOH, if my structure is different from the solution structure, would it be wrong? Or would I have to find the formal charges ...
- Mon Oct 28, 2019 8:34 pm
- Forum: Lewis Structures
- Topic: 2B. 1C
- Replies: 5
- Views: 180
2B. 1C
The problem asks you to draw the Lewis structure for ONF. So, the structure has N as the central atom, with a double bond between O and N and a single bond between N and F. But why can't you put the double bond between N and F and the single bond between O and N?
- Sun Oct 27, 2019 3:44 pm
- Forum: Resonance Structures
- Topic: Resonance hybrids
- Replies: 3
- Views: 108
Re: Resonance hybrids
A resonance hybrid is a blending of the Lewis structures for a molecule. For example, with the nitrate ion, you have 3 possible Lewis structures where the double bonds are in different positions. However, instead of only having one structure represent the nitrate ion (NO3-), it's better to have a re...
- Sun Oct 27, 2019 3:38 pm
- Forum: Lewis Structures
- Topic: Ionic vs. Covalent Lewis Structures
- Replies: 2
- Views: 673
Ionic vs. Covalent Lewis Structures
What is the difference between drawing Lewis structures for an ionic vs covalent compound? Is it only adding or subtracting the electrons for a cation or anion, respectively?
- Sun Oct 27, 2019 3:35 pm
- Forum: Lewis Structures
- Topic: Multiple Central Atoms
- Replies: 2
- Views: 171
Multiple Central Atoms
In a polyatomic atom, how would you know which atoms are the central atoms if there are multiple of them that could act as the central atoms? So an example in the book is CH3COOH, but how would you know how to make which atoms the central ones?
- Sun Oct 27, 2019 3:25 pm
- Forum: Ionic & Covalent Bonds
- Topic: ionization
- Replies: 5
- Views: 340
Re: ionization
Can someone please explain the concept of ionization? How do you know that ionization energy is based off the period table? Ionization energy is the energy required to remove an electron from the atom of a gas. The ionization energy is based off the periodic table because as you go from left to righ...
- Sun Oct 27, 2019 3:20 pm
- Forum: Ionic & Covalent Bonds
- Topic: Benzene's covalent bond
- Replies: 2
- Views: 204
Re: Benzene's covalent bond
Benzene's usual structure is a 6-carbon ring structure with 3 double bonds. It can also be written as a line structure. But what's special about benzene is that, according to the textbook, the line structure doesn't fit its experimental evidence. For instance, the C-C bonds in benzene are the same l...
- Sun Oct 20, 2019 7:39 pm
- Forum: Quantum Numbers and The H-Atom
- Topic: 1E. 1
- Replies: 4
- Views: 315
1E. 1
Which of the following increase when an electron in a lithium atom undergoes a transition from the 1s-orbital to a 2p-orbital? (a) Energy of the electron. (b) Value of n. (c) Value of l. (d) Radius of the atom. Which answers would be different for a hydrogen atom and in what way would they be differ...
- Sun Oct 20, 2019 7:33 pm
- Forum: Wave Functions and s-, p-, d-, f- Orbitals
- Topic: Hund's Rule
- Replies: 3
- Views: 190
Re: Hund's Rule
Hund's rule basically states that for a subshell with multiple orbitals, then you fill each orbital with one electron first before you fill a single orbital with two electrons. So for example, when you're filling in the p-orbitals, you fill up the px-, py-, and pz-orbitals with one electron each bef...
- Sun Oct 20, 2019 6:19 pm
- Forum: Electron Configurations for Multi-Electron Atoms
- Topic: 4s orbitals and 3d orbitals
- Replies: 2
- Views: 118
4s orbitals and 3d orbitals
In the book, it says "Argon completes the third period. From Fig. 1E.1, you can see that the energy of the 4s-orbital is slightly lower than that of the 3d-orbitals. As a result, instead of electrons entering the 3d-orbitals, the fourth period now begins by filling the 4s-orbitals..." How ...
- Sun Oct 20, 2019 2:54 am
- Forum: Wave Functions and s-, p-, d-, f- Orbitals
- Topic: 1D. 23
- Replies: 4
- Views: 264
Re: 1D. 23
So for c, would that mean there is only one orbital for the given quantum numbers?
- Sun Oct 20, 2019 2:41 am
- Forum: Wave Functions and s-, p-, d-, f- Orbitals
- Topic: 1D. 23
- Replies: 4
- Views: 264
1D. 23
How many orbitals can have the following quantum numbers in an atom:
(a) n=2, l=1
(b) n=4, l=2, ml= -2
(c) n=2
(d) n=3, l=2, ml= +1?
For (b) and (c), how does the magnetic quantum number tell you how many orbitals there are? I don't understand how to find the number of orbitals with ml.
(a) n=2, l=1
(b) n=4, l=2, ml= -2
(c) n=2
(d) n=3, l=2, ml= +1?
For (b) and (c), how does the magnetic quantum number tell you how many orbitals there are? I don't understand how to find the number of orbitals with ml.
- Thu Oct 17, 2019 12:50 am
- Forum: Properties of Electrons
- Topic: 1B. 15C [ENDORSED]
- Replies: 4
- Views: 269
1B. 15C [ENDORSED]
The velocity of an electron that is emitted from a metallic surface by a photon is 3.63 x 10^3 km/s. (a) What is the wavelength of the ejected electron? (b) No electrons are emitted from the surface of the metal until the frequency of the radiation reaches 2.50 x 10^16 Hz. How much energy is require...
- Sun Oct 13, 2019 8:32 pm
- Forum: Properties of Electrons
- Topic: constructive vs destructive interference
- Replies: 3
- Views: 238
Re: constructive vs destructive interference
Constructive interference refers to waves in phase. This means that the peak of one wave interacts with the peak of another, and the trough of one wave interacts with the trough of another. Thus, the resulting wave is larger in the sense that the trough is deeper and the peak is higher. Destructive ...
- Sun Oct 13, 2019 8:10 pm
- Forum: Properties of Light
- Topic: 1B #3
- Replies: 1
- Views: 78
Re: 1B #3
The answer is photoelectric effect. Light is used to eject electrons from a metal, and if light acted as a wave, then increasing the intensity of the light would mean more electrons being ejected. However, this didn't work. Instead, decreasing the wavelength allowed the ejection of electrons, which ...

