Search found 101 matches
- Sun Mar 15, 2020 1:37 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Galvanic and voltaic cells
- Replies: 5
- Views: 428
Re: Galvanic and voltaic cells
Galvanic and voltaic cells are the same.
- Sun Mar 15, 2020 1:35 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: calculating standard cell potential
- Replies: 6
- Views: 558
Re: calculating standard cell potential
You would take take the standard potential of the cathode and subtract the standard potential of the anode from it.
- Sun Mar 15, 2020 1:34 pm
- Forum: Arrhenius Equation, Activation Energies, Catalysts
- Topic: heterogeneous vs homogeneous catalysts
- Replies: 2
- Views: 227
Re: heterogeneous vs homogeneous catalysts
I don't think knowing the difference is too significant to this course and the final. I think it is just good to know that there are different kinds of catalysts.
- Sun Mar 15, 2020 1:33 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Chem Final Typo
- Replies: 8
- Views: 829
Re: Chem Final Typo
I felt that there was a typo, but maybe it was just Professor Lavelle's way of making sure we pay attention to the answers.
- Sun Mar 15, 2020 1:31 pm
- Forum: Method of Initial Rates (To Determine n and k)
- Topic: Graphs
- Replies: 3
- Views: 357
Re: Graphs
The graphs only show order in relation to a specific molecule/reactant. I do not believe we know the graphs for overall orders.
- Sun Mar 08, 2020 11:26 pm
- Forum: First Order Reactions
- Topic: Half Life
- Replies: 13
- Views: 845
Half Life
Why do we need to know how to calculate half lives? In what context would a half life question be given?
- Sun Mar 08, 2020 11:23 pm
- Forum: Second Order Reactions
- Topic: Derivation of the Equation
- Replies: 2
- Views: 252
Derivation of the Equation
Do we need to know how to derive the equation? I know that Professor Lavelle went over it in class, but I am not sure if we have to know how to derive it for the final.
- Sun Mar 08, 2020 11:22 pm
- Forum: Method of Initial Rates (To Determine n and k)
- Topic: Pre-Equilibrium
- Replies: 3
- Views: 298
Pre-Equilibrium
Why do we use the pre-equilbrium approach instead of the other methods presented in class?
- Sun Mar 08, 2020 11:20 pm
- Forum: Reaction Mechanisms, Reaction Profiles
- Topic: Relationship between Equilibrium Constant and Rate Constants
- Replies: 1
- Views: 285
Relationship between Equilibrium Constant and Rate Constants
How are equilibrium constants and rate constants related? I understand there is an equation relating them, but I would like a conceptual explanation as to how they are related.
- Sun Mar 08, 2020 11:17 pm
- Forum: Zero Order Reactions
- Topic: Order of Reaction
- Replies: 3
- Views: 335
Order of Reaction
How do you determine if a reaction is zero, first, or second order? Is there a way to tell without looking at a graph?
- Sun Mar 01, 2020 11:29 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Difference Between Galvanic and Voltaic Cells
- Replies: 3
- Views: 284
Difference Between Galvanic and Voltaic Cells
Is there a difference between galvanic and voltaic cells? Or are they just two names given to the same thing?
- Sun Mar 01, 2020 11:27 pm
- Forum: Balancing Redox Reactions
- Topic: Writing Half-reactions
- Replies: 1
- Views: 185
Re: Writing Half-reactions
I think that conventionally, you always add things together rather than subtracting them. Zn is being broken down into 2 electrons and Zn2+. Although it does seem clearer to subtract the electrons because it lost them, I would just stick with adding 2 electrons to Zn2+ to keep things consistent.
- Sun Mar 01, 2020 11:24 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Electromotive force
- Replies: 3
- Views: 243
Re: Electromotive force
Electromotive force is simply the potential difference between two cells. Electron flow can go in any direction, depending on which side has the cathode/anode. But, it is typically calculated cathode-anode (right minus left).
- Sun Mar 01, 2020 11:13 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Galvanic Cells and pH
- Replies: 1
- Views: 242
Re: Galvanic Cells and pH
I believe that it only matters when H+ or OH- are involved in the reaction (when you are using the Nernst equation).
- Sun Mar 01, 2020 11:07 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Cell Notation
- Replies: 4
- Views: 488
Re: Cell Notation
Since it is a solvent, you don't need to put it in the cell diagram.
- Sat Feb 22, 2020 5:13 pm
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: Nernst Equation and Le Chatelier's Principle
- Replies: 3
- Views: 554
Nernst Equation and Le Chatelier's Principle
Can someone summarize how le chatelier's principle and the nernst equation are related?
- Sat Feb 22, 2020 5:07 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Standard Cell Potentials
- Replies: 4
- Views: 300
Re: Standard Cell Potentials
Professor Lavelle says to do right minus left, because conventionally, the cathode is written on the right and the anode is written on the left. However, it should always be cathode-anode.
- Sat Feb 22, 2020 4:59 pm
- Forum: Balancing Redox Reactions
- Topic: Hw problem 6k.5 b)
- Replies: 2
- Views: 239
Re: Hw problem 6k.5 b)
I believe that you would assume that it is both an oxidizing and reducing agent. You should still split the reaction into 2 half reactions. Br2 --> Br- is the reduction reaction (0 to 1-) and Br2 --> BrO3- is the oxidation reaction (0 to 5+).
- Sat Feb 22, 2020 4:48 pm
- Forum: Balancing Redox Reactions
- Topic: Cell Diagrams
- Replies: 5
- Views: 312
Re: Cell Diagrams
Yes, Professor Lavelle said during lecture that when there are no conducting solids given in the problem (both the reactant and product are in solution), use Platinum as the electrode. He also said graphite works too but platinum is more common.
- Sat Feb 22, 2020 4:45 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: max potential in Galvanic Cell
- Replies: 4
- Views: 297
Re: max potential in Galvanic Cell
But why is it that the maximum potential of the cell is when the switch is closed (very little current flowing)? What exactly does "maximum potential" mean?
- Sun Feb 16, 2020 11:31 pm
- Forum: Entropy Changes Due to Changes in Volume and Temperature
- Topic: ΔS for Irreversible Expansion
- Replies: 3
- Views: 290
Re: ΔS for Irreversible Expansion
In the book, example 4I.3 explains this concept very well! I would go through that example and read some of the information in that section, as I believe the book explains delta S in more depth than what we covered in class.
- Sun Feb 16, 2020 11:29 pm
- Forum: Balancing Redox Reactions
- Topic: Oxidation Number Rules
- Replies: 7
- Views: 510
Oxidation Number Rules
Do we have to memorize the oxidation number rules?
- Sun Feb 16, 2020 11:21 pm
- Forum: Van't Hoff Equation
- Topic: Van't Hoff Equation
- Replies: 3
- Views: 303
Van't Hoff Equation
Can someone go over how the van't hoff equation was derived from delta g and how K plays a role in the equation? Thanks!
- Sun Feb 16, 2020 11:17 pm
- Forum: Van't Hoff Equation
- Topic: Assumptions
- Replies: 3
- Views: 279
Re: Assumptions
We always assume that delta S is the same at different temperatures because we can assume that the difference in delta S between the reactants and products is the same at two different temperatures.
- Sun Feb 16, 2020 11:09 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Spectator Ions
- Replies: 3
- Views: 198
Spectator Ions
Do we include spectator ions in the equilibrium constant?
- Sat Feb 08, 2020 3:14 pm
- Forum: Entropy Changes Due to Changes in Volume and Temperature
- Topic: Using Cv,m and Cp,m
- Replies: 2
- Views: 116
Using Cv,m and Cp,m
When do we use Cv,m and Cp,m? Also, why and when do we use 5R/2, 3R/2, etc.? I am kind of confused on this, as I don't recall Professor Lavelle really explaining it.
- Sat Feb 08, 2020 3:10 pm
- Forum: Phase Changes & Related Calculations
- Topic: Steam burns more than water
- Replies: 5
- Views: 256
Re: Steam burns more than water
Steam at 100C causes a worse burn than water at 100C because steam at 100C has a lot more energy than boiling water at the same temperature. If you recall the phase change graph, a lot of energy is put into vaporizing water into a gas. During a phase change, the temperature remains constant, but the...
- Sat Feb 08, 2020 3:06 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: 4B.3
- Replies: 1
- Views: 81
4B.3
4B.3: The internal energy of a system increased by 982 J when it was supplied with 492 J of energy as heat. (a) Was work done by or on the system? (b) How much work was done? I understand that work was done on the system for the internal energy to be increased by 982 J, but I don't understand why th...
- Sat Feb 08, 2020 2:59 pm
- Forum: Concepts & Calculations Using Second Law of Thermodynamics
- Topic: Entropy equations
- Replies: 2
- Views: 134
Re: Entropy equations
You can find a list of all the formulas and constants on the cover page of the first test we took. It has most of the formulas needed, but depending on the question, you may need to know how to manipulate equations to solve the question.
- Sat Feb 08, 2020 2:54 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: 4A.13
- Replies: 2
- Views: 137
Re: 4A.13
Since the calorimeter contains the same volume of liquid in both cases (0.200 L and 100.0 mL+100.0 mL), there is no change in volume and the final volume is constant at 0.100 L. That is why the volumes are not considered since there is no change in volume.
- Sat Feb 08, 2020 2:51 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: Delta U at Constant V/P
- Replies: 4
- Views: 224
Re: Delta U at Constant V/P
At constant volume, delta u = q because there is no work being done. Work requires that there is a change in volume, as the book defines work as "the process of achieving motion against an opposing force". At constant pressure, delta u = q - P*delta V because you can rearrange the equation...
- Wed Jan 29, 2020 10:34 am
- Forum: Ideal Gases
- Topic: ideal gases
- Replies: 14
- Views: 981
Re: ideal gases
An ideal gas is a theoretical gas that does not have any intermolecular attractive forces, does not take up space, and obeys the ideal gas law.
- Wed Jan 29, 2020 10:30 am
- Forum: Non-Equilibrium Conditions & The Reaction Quotient
- Topic: approximation
- Replies: 4
- Views: 198
Re: approximation
You can approximate x to be 0 when the Ka/Kb value is less than 10^-3. However, you also need to check that the x value you calculate is less than 5% of the initial amount.
- Wed Jan 29, 2020 10:29 am
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Bomb Calorimetry
- Replies: 2
- Views: 129
Re: Bomb Calorimetry
The bomb calorimeter is a constant volume calorimeter that is used to measure the heat of combustion of a reaction.
- Wed Jan 29, 2020 10:27 am
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Bomb calorimeter
- Replies: 2
- Views: 110
Re: Bomb calorimeter
A bomb calorimeter is a constant volume calorimeter. The reason why it is called a bomb calorimeter is because there is a large increase in pressure when a reaction occurs in the bomb vessel.
- Wed Jan 29, 2020 10:02 am
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Homework for Week 4
- Replies: 2
- Views: 152
Homework for Week 4
I am a bit confused on which homework problems we should be doing for this week. It seems like the homework problems that we could do are all over the place (some in 4A, 4C, 4D). Are these the sections we should be doing problems from or am I missing something?
- Sun Jan 26, 2020 11:54 pm
- Forum: Phase Changes & Related Calculations
- Topic: Chem 14A Final Pickup
- Replies: 8
- Views: 367
Re: Chem 14A Final Pickup
I have picked up the final, but I am unsure if they will still be available this week because Professor Lavelle's email said that they would be available during week 3.
- Sun Jan 26, 2020 11:53 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Methods to Calculate Enthalpies
- Replies: 6
- Views: 403
Methods to Calculate Enthalpies
How do we know which method to use when calculating the total enthalpy of a reaction?
- Sun Jan 26, 2020 11:51 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Method of calculating
- Replies: 3
- Views: 139
Re: Method of calculating
I believe using the standard reaction enthalpies would give you the most accurate answers. In the slides, it stated that if bond enthalpies are not available, then standard reaction enthalpies should be used.
- Sun Jan 26, 2020 11:46 pm
- Forum: Phase Changes & Related Calculations
- Topic: Hess's Law
- Replies: 5
- Views: 173
Re: Hess's Law
Hess's Law states that enthalpy change at each step of a reaction can be added together to give a total enthalpy change because enthalpy is a state function and is therefore additive.
- Sun Jan 26, 2020 11:44 pm
- Forum: Phase Changes & Related Calculations
- Topic: State Property
- Replies: 6
- Views: 202
Re: State Property
State properties are the same as state functions. The path that is taken to get from one point to another is not important. An example Professor Lavelle gave in class was altitude. Enthalpy is another example.
- Mon Jan 13, 2020 5:12 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: The Value of Kw
- Replies: 3
- Views: 176
The Value of Kw
Can the value of Kw ever change? I remember Professor Lavelle talking about temperature being able to change Kw but I don't really recall why or if temperature was the only factor that could change its value.
- Mon Jan 13, 2020 5:05 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Are Lectures Bruincasted?
- Replies: 10
- Views: 393
Re: Are Lectures Bruincasted?
I don't believe that lectures are bruincasted! They aren't found anywhere on his website.
- Mon Jan 13, 2020 4:12 pm
- Forum: Ideal Gases
- Topic: Kc and Kp
- Replies: 5
- Views: 265
Re: Kc and Kp
Kc and Kp both measure the equilibrium constant of a reaction. Kc is used when calculating in terms of concentration whereas Kp is used when calculating in terms of pressure. Typically, you would calculate the Kp of a reaction that is made up of all gases, but both Kp and Kc should be the same since...
- Mon Jan 13, 2020 4:05 pm
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: 5J.3
- Replies: 5
- Views: 211
Re: 5J.3
When you remove NO, the reaction favors the products because it wants to reach equilibrium. Since there is now less NO, and therefore products, the reaction proceeds forward to produce more products. This causes NH3 (reactant) to decrease.
- Mon Jan 13, 2020 4:02 pm
- Forum: Student Social/Study Group
- Topic: F19 Final
- Replies: 4
- Views: 178
Re: F19 Final
They will be available third week at 3034 Young Hall as per Professor Lavelle's email.
- Mon Jan 06, 2020 10:40 pm
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Pressure's Effect on Equilibrium
- Replies: 6
- Views: 262
Pressure's Effect on Equilibrium
Why is it that when pressure is increased by adding an inert gas to a reaction, the K value does not change? Also, what happens when pressure is decreased?
- Mon Jan 06, 2020 10:37 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: 5I.23
- Replies: 1
- Views: 98
Re: 5I.23
Hi! When you make your ICE table, the x value that you use to add and subtract to and from the reactants/products is actually equal to .478 M, which was given in the original problem because it is given that the equilibrium concentration (the E section of the ICE table) is 0.478 M.
- Mon Jan 06, 2020 10:25 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Equilibrium Constant for Solids/Gases
- Replies: 5
- Views: 227
Re: Equilibrium Constant for Solids/Gases
You do take solids and liquids out of the equilibrium constant equation, but not including them does not eliminate the numerator. A quick google search indicated that the activity (I believe Professor Lavelle briefly mentioned that we would be considering concentrations to be the same as the activit...
- Mon Jan 06, 2020 10:22 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Module: Equilibrium Part 3 Question 17
- Replies: 3
- Views: 152
Re: Module: Equilibrium Part 3 Question 17
Remember that when solving a quadratic equation, x will have two values. The other value that you have not yet solved for will probably give you the right answer to the question! (Your quadratic equation is correct, you just need to solve for the other x).
- Mon Jan 06, 2020 10:20 pm
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Equilibrium and Gases
- Replies: 3
- Views: 188
Re: Equilibrium and Gases
When pressure is increased by decreasing the volume, equilibrium shifts to to the side with less moles of gas because the concentrations of each compound is being increased according to the equation P=n/v. If pressure is increased by decreasing the volume, then the concentration increases since v is...
- Wed Dec 04, 2019 12:27 pm
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: Drawing structures on the final
- Replies: 3
- Views: 204
Re: Drawing structures on the final
I don't believe you need to know how to draw these compounds. I think you just need to know how to name/identify them.
- Wed Dec 04, 2019 12:25 pm
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: Videos on polydentate ligands?
- Replies: 2
- Views: 283
Re: Videos on polydentate ligands?
I think that this video is pretty helpful! https://www.youtube.com/watch?v=r4H5XjJPn58. Hope this helps :)
- Wed Dec 04, 2019 12:20 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Final Review Session
- Replies: 6
- Views: 418
Re: Final Review Session
Yes! Lyndon, one of the UAs, posted another review packet under the name "Marshmallow". There is also a chemistry review session this Friday!
- Wed Dec 04, 2019 12:18 pm
- Forum: Identifying Acidic & Basic Salts
- Topic: pH of salt solutions
- Replies: 2
- Views: 154
pH of salt solutions
Why do certain ions in salts not affect the pH of the solution?
- Wed Dec 04, 2019 12:11 pm
- Forum: Acidity & Basicity Constants and The Conjugate Seesaw
- Topic: 6.4
- Replies: 1
- Views: 191
Re: 6.4
The pH of pure water is 7. The equation to find pH is pH=-log[H3O+]. Substituting 7 into the equation, you would find that the concentration of hydronium ions is 1*10^-7. Then, to find the number of ions present, you would then do the following: \frac{1*10^-7mol}{L} (\frac{0.100L}{1})(\f...
- Wed Dec 04, 2019 12:05 pm
- Forum: Hybridization
- Topic: Hybridization of terminal atoms
- Replies: 2
- Views: 140
Re: Hybridization of terminal atoms
No, I don't believe you need to know the hybridization of terminal atoms. I believe you just need to know the hybridization of central atoms.
- Tue Nov 26, 2019 9:44 am
- Forum: Administrative Questions and Class Announcements
- Topic: Final Exam Content
- Replies: 14
- Views: 796
Re: Final Exam Content
I believe that the final exam will cover everything we learned this quarter. Just as how the midterm also covered the high school material that we covered in the first two weeks, the final will probably be similarly formatted and pull questions from the different topics we learned this quarter.
- Tue Nov 26, 2019 9:38 am
- Forum: Bronsted Acids & Bases
- Topic: Lewis Structures of Acid/Base reactions
- Replies: 3
- Views: 185
Re: Lewis Structures of Acid/Base reactions
I don't believe you would have to know how to draw out the lewis structures for acid/base reactions! However, I feel that knowing the lewis structures of acid/base reactions will strengthen and further your understanding of how these reactions work.
- Tue Nov 26, 2019 9:34 am
- Forum: Bronsted Acids & Bases
- Topic: Equlibrium Constant Expression for Strong Acids/Bases
- Replies: 3
- Views: 150
Re: Equlibrium Constant Expression for Strong Acids/Bases
Ka and Kb don't exist for strong acids and bases because they dissociate completely in solution. If you recall the formula for calculating the equilibrium constants, K_{a}=\frac{[H^{+}][A^{-}]}{[HA]} you will see that the concentration of the acid itself is in the denominator. Since strong acids/bas...
- Tue Nov 26, 2019 9:24 am
- Forum: Sigma & Pi Bonds
- Topic: Strength of the Bonds
- Replies: 2
- Views: 139
Re: Strength of the Bonds
Sigma bonds are actually stronger than pi bonds. Sigma bonds have more of a linear overlap whereas pi bonds have a "parallel" overlap. The electrons are held more tightly to the nuclei of sigma bonds than pi bonds. https://chem.libretexts.org/@api/deki/files/78352/CK12_Screenshot_9-20-3.pn...
- Tue Nov 26, 2019 9:20 am
- Forum: Dipole Moments
- Topic: Intermolecular force
- Replies: 5
- Views: 614
Re: Intermolecular force
Yes! It would be perfectly fine to refer to intermolecular forces as stronger or weaker. It is true that the stronger the intermolecular force is, the harder it is to break the bonds between the molecules.
- Sun Nov 24, 2019 11:57 pm
- Forum: *Molecular Orbital Theory (Bond Order, Diamagnetism, Paramagnetism)
- Topic: bonds
- Replies: 2
- Views: 202
Re: bonds
It can be nonpolar if the dipole moments cancel each other out. For example, carbon dioxide has polar bonds but the overall molecule is nonpolar. The dipole moments face each other and the molecule is also symmetrical, cancelling each other out and making the molecule nonpolar.
- Sun Nov 24, 2019 11:55 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: electron and molecular geometry of H20
- Replies: 3
- Views: 242
Re: electron and molecular geometry of H20
The electron geometry of H2O is tetrahedral because of the two sets of lone pairs. Therefore, its molecular geometry is bent (AX2E2).
- Sun Nov 24, 2019 11:54 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Molecular shape of carbon Dioxide
- Replies: 10
- Views: 635
Re: Molecular shape of carbon Dioxide
The molecular shape of carbon dioxide is bent because it has no lone pairs.
- Sun Nov 24, 2019 11:47 pm
- Forum: Bond Lengths & Energies
- Topic: Polarity
- Replies: 5
- Views: 591
Re: Polarity
Polar molecules tend to have higher boiling/melting points because they have greater intermoleculr forces than nonpolar molecules do. Nonpolar molecules only have van der Waals forces acting between molecules., which is the weakest form of intermolecular force. Polar molecules have dipoles that give...
- Sun Nov 24, 2019 11:44 pm
- Forum: Hybridization
- Topic: Sigma and Pi Bonds
- Replies: 21
- Views: 1067
Re: Sigma and Pi Bonds
In a molecule, you can identify sigma and pi bonds by counting the number of single, double, and triole bonds it has. Every single bond is a sigma bond, every double bond has 1 sigma bond and 1 pi bond, and every triple bond has 1 sigma bond and 2 pi bonds.
- Sat Nov 16, 2019 10:17 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: dipole-dipole
- Replies: 3
- Views: 256
Re: dipole-dipole
The OH group on the molecule is what makes the molecule polar. Its shape is a tetrahedral and the OH group is much more negative than the rest of the molecule.
- Sat Nov 16, 2019 10:12 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Dipole moments
- Replies: 2
- Views: 189
Re: Dipole moments
You would add an arrow. Make sure the bonds in the lewis structure are just lines!
- Sat Nov 16, 2019 10:10 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Bond Angles
- Replies: 4
- Views: 272
Re: Bond Angles
Sometimes, the angles can differ slightly because of how electronegative the atoms are in a molecule. If an atom is more electronegative, then the bond angle may be reduced.
- Sat Nov 16, 2019 10:05 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Subscript on E
- Replies: 5
- Views: 316
Re: Subscript on E
Hi!
You can write it however you want :) they are the same thing, but putting a subscript of 1 may make it clearer for the person grading the exams.
You can write it however you want :) they are the same thing, but putting a subscript of 1 may make it clearer for the person grading the exams.
- Sat Nov 16, 2019 10:03 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Bent and Angular?
- Replies: 6
- Views: 374
Re: Bent and Angular?
Bent and angular are synonymous but typically, bent is used more often!
- Fri Nov 08, 2019 12:41 pm
- Forum: Photoelectric Effect
- Topic: frequency and ejection of electrons
- Replies: 7
- Views: 795
Re: frequency and ejection of electrons
The photoelectric effect states that there is a certain amount of energy (threshold energy) needed for an electron to be ejected. The frequency of the incoming photon is what determines whether an electron can be ejected. If the photon has a high enough frequency so that its energy surpasses that of...
- Fri Nov 08, 2019 12:33 pm
- Forum: DeBroglie Equation
- Topic: Light waves
- Replies: 2
- Views: 227
Re: Light waves
You can only use c=(wavelength)(frequency) and E=h(frequency) for light waves. De Broglie's equation is used only for things with mass, and light does not have mass. Therefore, De Broglie can not be used for light waves.
- Fri Nov 08, 2019 12:27 pm
- Forum: Interionic and Intermolecular Forces (Ion-Ion, Ion-Dipole, Dipole-Dipole, Dipole-Induced Dipole, Dispersion/Induced Dipole-Induced Dipole/London Forces, Hydrogen Bonding)
- Topic: Interaction Potential Energy
- Replies: 3
- Views: 226
Re: Interaction Potential Energy
Alpha represents the polarizability of an atom and depends on the number of electrons and size of the atom. R in the equation represents the radius. So, according to the equation, the interaction potential energy is proportional to 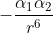 .
.
- Fri Nov 08, 2019 12:23 pm
- Forum: DeBroglie Equation
- Topic: De Broglie Equation Derivation and Use
- Replies: 7
- Views: 1176
Re: De Broglie Equation Derivation and Use
We don't necessarily need to know how to derive De Broglie's Equation. Just know that De Broglie's Equation is used when calculating the wavelength or frequency of anything with mass, such as electrons.
- Sun Nov 03, 2019 5:18 pm
- Forum: Polarisability of Anions, The Polarizing Power of Cations
- Topic: polarizing power
- Replies: 3
- Views: 229
Re: polarizing power
Large distortion refers to the distortion of the electron cloud when an ionic compound is formed. The cation in the ionic compound pulls the anion's electrons closer to itself, distorting the electron cloud in the direction of the cation. That is why small cations have such high polarizing power.
- Sun Nov 03, 2019 5:13 pm
- Forum: Polarisability of Anions, The Polarizing Power of Cations
- Topic: polarizing power vs. polarizability
- Replies: 1
- Views: 171
Re: polarizing power vs. polarizability
Polarizing power is the ability of an atom/ion to cause large distortions in the electron cloud of another, whereas polarizability refers to atoms or ions that readily undergo large distortions, such as iodide. Iodide is highly polarizable because it has a low effective nuclear charge and its outerm...
- Sun Nov 03, 2019 5:11 pm
- Forum: Lewis Structures
- Topic: structure stability
- Replies: 2
- Views: 168
Re: structure stability
Bond order is simply the number of bonds that link a specific pair of atoms (from the textbook). For example, the bond order in the molecule is three, since they are bound by a triple bond. To determine the most stable structure, you should try to get the formal charges on each atom of the molecule ...
- Sun Nov 03, 2019 5:03 pm
- Forum: Electron Configurations for Multi-Electron Atoms
- Topic: Electron Configuration Pattern?
- Replies: 1
- Views: 193
Re: Electron Configuration Pattern?
Hi! There's actually a really handy diagram that helps you determine electron configuration of an atom much easier. https://www.google.com/url?sa=i&source=images&cd=&ved=2ahUKEwi5gaHKrM_lAhUToZ4KHZjxBGwQjRx6BAgBEAQ&url=https%3A%2F%2Fwww.pinterest.com%2Fpin%2F326511041712732752%2F&...
- Fri Nov 01, 2019 2:05 pm
- Forum: Resonance Structures
- Topic: Resonance
- Replies: 4
- Views: 182
Re: Resonance
I don't believe that resonance structures are more reactive. In fact, I believe thay resonance actually stabilizes molecules because it delocalizes the molecule and lowers the overall energy. The electrons have a greater space to occupy.
- Sun Oct 27, 2019 3:25 pm
- Forum: Ionic & Covalent Bonds
- Topic: ionization
- Replies: 5
- Views: 340
Re: ionization
Ionization energy is the energy needed to remove an electron from an atom. It has a trend in the periodic table because as you move across a period from left to right, the ionization energy increases, and as you move up a group the ionization energy also increases. It increases from left to right be...
- Sun Oct 27, 2019 3:18 pm
- Forum: Trends in The Periodic Table
- Topic: Ionization and Electron Affinity
- Replies: 5
- Views: 337
Re: Ionization and Electron Affinity
Ionization energy refers to the energy needed to remove an electron from an atom (in its gas phase) while electron affinity refers to the energy released when an electron is added to an atom (in its gas phase). Electron affinity could also be explained as how much an atom attracts/gains electrons!
- Sun Oct 27, 2019 3:13 pm
- Forum: Trends in The Periodic Table
- Topic: Central Atom
- Replies: 13
- Views: 536
Re: Central Atom
The atom with the lowest ionization energy is commonly used as the central atom when drawing lewis structures! Dr. Lavelle talked about this briefly in lecture :)
- Sun Oct 27, 2019 3:11 pm
- Forum: Quantum Numbers and The H-Atom
- Topic: 1D.23
- Replies: 1
- Views: 85
Re: 1D.23
For b, there is actually only 1 orbital because n=4 refers to the 4th shell, l=2 refers to the d subshell, and m(l)=-2 specifies a single orbital. Therefore, part b only has 1 orbital. For part c, there are 4 orbitals because n=2 refers to the 2nd shell. In the second shell, the s and p subshells ex...
- Sun Oct 27, 2019 2:55 pm
- Forum: Resonance Structures
- Topic: Bond lenghts.
- Replies: 11
- Views: 412
Re: Bond lenghts.
I don't believe that we have to know how to find specific bond lengths. We just have to understand the differences in lengths of double and single bonds. Bond lengths within molecules with resonance must also be considered. The bond lengths in molecules with resonance are more like an "average&...
- Sun Oct 20, 2019 5:35 pm
- Forum: Quantum Numbers and The H-Atom
- Topic: 1D.23
- Replies: 1
- Views: 95
Re: 1D.23
Yes, your answer would be a summation of all the possible values for m and l given that n=2. If n=2, l must be 0 or 1 and m could therefore be -1, 0, or 1 if l=1. If l=0, then m could only be 0. The total number of orbitals then would be 4.
- Sun Oct 20, 2019 5:28 pm
- Forum: Electron Configurations for Multi-Electron Atoms
- Topic: Electron Spin
- Replies: 2
- Views: 102
Re: Electron Spin
When two electrons are paired, it means that they exist in the same orbital within a subshell. When electrons are parallel, they are filling the orbitals singly before pairing. According to Hund's Rule, this happens because when there are two or more orbitals with equal energies, electrons will fill...
- Sun Oct 20, 2019 5:19 pm
- Forum: DeBroglie Equation
- Topic: 1B.21
- Replies: 3
- Views: 242
Re: 1B.21
This question is dealing with deBroglie's equation, as it is asking for the wavelength of something that has mass and velocity (momentum). Since the units are not what we want, we must use dimensional analysis to convert ounces to kilograms and miles per hour to meters per second. It is given that o...
- Sun Oct 20, 2019 5:09 pm
- Forum: DeBroglie Equation
- Topic: DeBroglie Equation
- Replies: 11
- Views: 375
Re: DeBroglie Equation
You would use the DeBroglie equation when trying to determine the wavelength of anything with momentum (mass * velocity). It is used in particular for items with very small masses, such as electrons. Typically, when the question is asking about the wavelength or velocity of an electron or a small pa...
- Sun Oct 20, 2019 5:04 pm
- Forum: Wave Functions and s-, p-, d-, f- Orbitals
- Topic: Orbitals
- Replies: 8
- Views: 314
Re: Orbitals
The reason why Scandium is written as [Ar] 3d1 4s2 is because after the 20th element in the periodic table (Calcium), the 3d orbitals have a slightly lower energy than the 4s orbitals. Therefore, you would start filling the 3d orbitals until it is full. The 4s orbital is filled first because it is i...
- Fri Oct 11, 2019 12:51 pm
- Forum: Empirical & Molecular Formulas
- Topic: Molecular formula
- Replies: 6
- Views: 2921
Re: Molecular formula
You would first need to find the empirical formula using the mass percentages. You would assume that there is 100g of the molecule and convert the grams of each atom into moles. Then, you would divide the moles by the smallest number of moles to find the ratio of atoms in the molecule. To find the m...
- Fri Oct 11, 2019 12:47 pm
- Forum: SI Units, Unit Conversions
- Topic: How to express answers
- Replies: 13
- Views: 512
Re: How to express answers
I don't believe that sig figs are too important in this class. My TA told us that sig figs are not too big of a deal, as long as you do not round to whole numbers or to only 1 decimal place when the answer should clearly have 2 or 3 decimal places. As for scientific notation, I believe that it is mo...
- Fri Oct 11, 2019 12:38 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Posts
- Replies: 6
- Views: 290
Re: Posts
The 5 homework questions are always due in your discussion! As for the 5 posts for chemistry community, you need to have all your posts published by 11:59 PM Sunday night. I recommend that you contribute to discussions asking more conceptual questions, rather than homework problems. I feel that it i...
- Fri Oct 11, 2019 12:20 pm
- Forum: Empirical & Molecular Formulas
- Topic: Decimals to Whole Numbers
- Replies: 6
- Views: 558
Re: Decimals to Whole Numbers
I believe that rounding from 3.1 to 3 would be okay if you are solving for empirical formulas. If the number is about .1 away from a whole number, I don't believe that it would hurt to round it to a whole number. However, rounding to whole numbers in other problems besides solving for empirical/mole...
- Fri Oct 11, 2019 12:08 pm
- Forum: General Science Questions
- Topic: Balancing Chemical Equations
- Replies: 5
- Views: 468
Re: Balancing Chemical Equations
This reaction is a combustion reaction, so O2 would be one of the reactants. Therefore, the chemical equation would be O2 + C7H16 -> CO2 + H2O. You would first balance the carbon, so you would put 7 as the stoichiometric coefficient in front of the carbon dioxide on the products side. Then, you woul...
- Thu Oct 03, 2019 10:45 pm
- Forum: SI Units, Unit Conversions
- Topic: Advice
- Replies: 3
- Views: 206
Re: Advice
You just need to use dimensional analysis! Dimensional analysis utilizes the cancellation of units.
- Thu Oct 03, 2019 10:37 pm
- Forum: SI Units, Unit Conversions
- Topic: Mass % comp accuracy F5
- Replies: 5
- Views: 358
Re: Mass % comp accuracy F5
Yes, I believe it's totally fine! It depends on the periodic table you are using too. Some periodic tables round off at different sig figs so you should be fine.
- Wed Oct 02, 2019 1:29 am
- Forum: Empirical & Molecular Formulas
- Topic: Homework problem E3 [ENDORSED]
- Replies: 1
- Views: 156
Re: Homework problem E3 [ENDORSED]
I believe this question is simpler than what it seems. In the question, the gallium atoms are literally represented by the blue circles on the scale. If you count them, you would see that there are 9 "atoms" of gallium on the left hand side of the scale. Comparing the molar masses of galli...
- Wed Oct 02, 2019 1:24 am
- Forum: Molarity, Solutions, Dilutions
- Topic: G 25
- Replies: 7
- Views: 377
Re: G 25
Since you are doubling the volume of the solution 90 times, the final volume would be much greater than the initial volume. The question asks how many molecules would be present in only 10. mL of the final solution. Since the volume has increased so dramatically, 10. mL of the final solution would c...

