Search found 71 matches
- Tue Mar 17, 2020 12:43 am
- Forum: Administrative Questions and Class Announcements
- Topic: Saying Thank You to Dr. Lavelle
- Replies: 490
- Views: 547475
Re: Saying Thank You to Dr. Lavelle
Thank you for all of the hard work that you do for us students, especially after all the recent cancellations. You've been a great professor these last two quarters and it's very apparent that you want us to succeed, from organizing the peer learning sessions to creating and moderating Chemistry Com...
- Sat Mar 14, 2020 5:51 pm
- Forum: General Rate Laws
- Topic: Coefficients and Rate constants
- Replies: 2
- Views: 244
Re: Coefficients and Rate constants
If it helps, this is the equation for the unique reaction rate that is in the textbook (7th edition, pg. 590): aA+bB\rightarrow cC+dD Unique average reaction rate = -\frac{1}{a}\frac{\Delta [A]}{\Delta t}=-\frac{1}{b}\frac{\Delta [B]}{\Delta t}=\frac{1}{c}\frac{\Delta [C]}{\Delta t}=\frac{1}{d}\frac...
- Sat Mar 14, 2020 1:07 pm
- Forum: Acidity & Basicity Constants and The Conjugate Seesaw
- Topic: Ka and Kb
- Replies: 4
- Views: 513
Ka and Kb
Just to review, Ka is used for acidic reactions that result in H+ and H3O+, while Kb is for basic reactions that result in OH-, right?
- Thu Mar 12, 2020 11:11 am
- Forum: Administrative Questions and Class Announcements
- Topic: Final
- Replies: 12
- Views: 828
Re: Final
Will there be a time limit on the test? Like he releases it at a certain time and we have to turn it in 3 hours later?
- Tue Mar 10, 2020 11:49 pm
- Forum: Administrative Questions and Class Announcements
- Topic: What is the plan for the final?
- Replies: 16
- Views: 1055
Re: What is the plan for the final?
Has anyone ever taken a take-home final before? What are they like?
- Sat Mar 07, 2020 11:11 am
- Forum: Reaction Mechanisms, Reaction Profiles
- Topic: Slow step
- Replies: 4
- Views: 344
Re: Slow step
According to some other posts, you just have to look at the individual and overall rate laws. The step with the same rate law as the overall rate law is the slowest step.
- Sat Mar 07, 2020 10:48 am
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: Nernst Equation for Concentration Cells
- Replies: 6
- Views: 405
Re: Nernst Equation for Concentration Cells
How do you know what n is when the half reactions have different numbers of electrons being transferred? Would you balance them and use that number? Yeah, just find a common multiple for both of them and that's n For example, if one half reaction is transferring 4 electrons and the other 2, you can...
- Sat Mar 07, 2020 10:37 am
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Determining the cathode/anode
- Replies: 9
- Views: 784
Re: Determining the cathode/anode
Since the half reactions are usually given to us as reductions, the standard potential of the cathode should be greater than the anode. This is because E^{\circ} is the standard potential of the half reaction. The greater it is, the more likely it is that the reaction will occur. We are always given...
- Fri Mar 06, 2020 8:08 am
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: Equilibrium Constants
- Replies: 3
- Views: 257
Re: Equilibrium Constants
Yeah, there are plenty of equations that you can use depending on what values you're given, like E°cell = E°cathode − E°anode or the Nernst equation.
- Fri Mar 06, 2020 7:50 am
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: Finding Q
- Replies: 7
- Views: 555
Re: Finding Q
Juana Abana 1G wrote:So then the anode is always the product and the cathode is always the reactant?
In most cases, yes, but I would always write out the reaction to be sure.
- Fri Mar 06, 2020 7:45 am
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: Nernst Equation for Concentration Cells
- Replies: 6
- Views: 405
Re: Nernst Equation for Concentration Cells
In concentrations cells, the two electrodes are the same except for their concentrations. Because the molecules are the same, the reactions on either side are the reverse reaction of the other, so E cell will always be 0 because E cathode and E anode cancel out. So in the equation E_{cell}=E^{\circ}...
- Sun Mar 01, 2020 9:58 pm
- Forum: Balancing Redox Reactions
- Topic: reversible reactions
- Replies: 2
- Views: 250
Re: reversible reactions
Oxidation only occurs at the anode and reduction at the cathode, so just check whether electrons are being added or removed for each reaction.
- Sun Mar 01, 2020 9:46 pm
- Forum: Work, Gibbs Free Energy, Cell (Redox) Potentials
- Topic: identifying cathode/anode
- Replies: 4
- Views: 380
Re: identifying cathode/anode
Both shouldn't be decreasing since a galvanic cell requires oxidation at the anode and reduction at the cathode. If both species have negative oxidation numbers, then they are both gaining electrons and being reduced. In that case, the cell wouldn't function properly because there is no source of el...
- Sun Mar 01, 2020 9:24 pm
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: Concentration Cells
- Replies: 4
- Views: 333
Re: Concentration Cells
While galvanic cells have different ions reacting on the cathode and anode, concentration cells have the same species on either side, but the concentrations are different. That's why it's called a concentration cell. The standard cell potential of a concentration cell is 0 because the reactions are ...
- Sun Mar 01, 2020 10:43 am
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: Electrolysis
- Replies: 3
- Views: 284
Re: Electrolysis
The negative and positive signs around the anode and cathode on galvanic cell drawings represent the direction that electrons are going. Electrons flow from the negative to positive pole. I think the signs represent the potentials of either side, but I'm pretty sure you don't need to know this.
- Thu Feb 27, 2020 5:45 pm
- Forum: Balancing Redox Reactions
- Topic: REDOX Agents
- Replies: 9
- Views: 783
Re: REDOX Agents
When something is an agent, they are usually doing the thing they are described as. For example, a cleaning agent cleans. In the same way, an oxidizing agent oxidizes another molecule, which leads to the agent being reduced. A reducing agent reduces, which causes it to be oxidized in the end.
- Sat Feb 22, 2020 11:04 am
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Pt
- Replies: 7
- Views: 480
Re: Pt
Pt meant platinum and it was used as an inert conductor/electrode to transfer electrons. We include it in the cell diagram on the cathode side. But some problems in the textbook have Pt(s) on the anode side or both sides (6L.3 (d) and (e)). Why do some have it only on the cathode or anode side and ...
- Sat Feb 22, 2020 10:45 am
- Forum: Balancing Redox Reactions
- Topic: Charge of oxygen
- Replies: 15
- Views: 756
Re: Charge of oxygen
In most gases, such as H2 or O2, I'm pretty sure the oxidation number is 0, or else it would be shown having a charge.
- Sat Feb 22, 2020 10:41 am
- Forum: Work, Gibbs Free Energy, Cell (Redox) Potentials
- Topic: cell potential equation
- Replies: 5
- Views: 367
Re: cell potential equation
F is Faraday's constant, the charge per mole of electrons. It is 9.648533... x 10 4 C/mol, or 96,485 C/mol E CELL knot will sometimes be given, or we'll need to calculate it through the equation E_{CELL}^{\circ}=E^{\circ}_{Cathode}-E^{\circ}_{Anode} . In this case, we will be given the E of the cath...
- Sat Feb 22, 2020 10:30 am
- Forum: Work, Gibbs Free Energy, Cell (Redox) Potentials
- Topic: How to find n in ΔG°= -nFE°
- Replies: 2
- Views: 278
Re: How to find n in ΔG°= -nFE°
n is the amount of electrons being transferred, so you just need to find that, which we've been doing with half-reactions. You can split up the redox reaction into 2 half-reactions. In 2Ce^{4+}(aq)\rightarrow 2Ce^{3+}(aq) , you can tell that there are 2 electrons being added. In 3I^{...
- Sat Feb 22, 2020 10:21 am
- Forum: Work, Gibbs Free Energy, Cell (Redox) Potentials
- Topic: n in -nFE
- Replies: 12
- Views: 881
Re: n in -nFE
In the 7th edition of the textbook, the equation is explained on pg. 547. Basically, the change in Gibbs free energy equals the max nonexpansion work of an isobaric and isothermal reaction ( \Delta G=w_{e, max} ). Work is done when electrons move through a potential difference, which it can be calcu...
- Thu Feb 20, 2020 3:41 pm
- Forum: Balancing Redox Reactions
- Topic: Oxidizing/reducing agent
- Replies: 7
- Views: 534
Re: Oxidizing/reducing agent
Focus on the word "agent." The agent is the one that does the action. So an oxidizing agent oxidizes another molecule. It passes its oxidized status to another molecule and it becomes reduced. A reducing agent reduces another molecule and becomes oxidized. If that's too confusing, just kno...
- Sun Feb 16, 2020 3:54 pm
- Forum: Biological Examples (*DNA Structural Transitions, etc.)
- Topic: organic reactions, and environmental and biological examples
- Replies: 4
- Views: 764
Re: organic reactions, and environmental and biological examples
I don't remember any examples he gave in lecture, but in the other topic about the environment and fossil fuels, which is also under Thermodynamics, he talks about how burning wood, natural gas, and other resources are exothermic and therefore used as energy sources. They also cause acid rain.
- Thu Feb 13, 2020 6:51 pm
- Forum: Entropy Changes Due to Changes in Volume and Temperature
- Topic: Units in Entropy Equation for Volume
- Replies: 5
- Views: 349
Units in Entropy Equation for Volume
When using the equation ) , can nR be substituted with
, can nR be substituted with 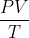 because of
because of 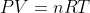 or do the units not work out? Entropy is written with joules, right? And there was a conversion between L.atm and J that we always needed to do in HW problems.
or do the units not work out? Entropy is written with joules, right? And there was a conversion between L.atm and J that we always needed to do in HW problems.
- Thu Feb 13, 2020 6:43 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: equilibrium concentrations
- Replies: 7
- Views: 523
Re: equilibrium concentrations
You calculate the concentrations in mol/L, but the equilibrium constant has no units.
- Wed Feb 12, 2020 3:41 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: enthalpy constant pressure
- Replies: 1
- Views: 109
Re: enthalpy constant pressure
The textbook has a section explaining this (pg. 263 in 7th edition) Basically, the full equation is \Delta H = \Delta U+P\Delta V You can simplify this by swapping in other equations: \Delta H=q+w+P\Delta V \Delta H = q-P\Delta V+P\Delta V The PVs cancel out, giving us: \Delta H=q Remember that this...
- Wed Feb 12, 2020 11:56 am
- Forum: Thermodynamic Systems (Open, Closed, Isolated)
- Topic: Examples of systems
- Replies: 3
- Views: 108
Re: Examples of systems
Energy is usually exchanged through heat. So heat can be transferred in both open and closed systems, but in isolated systems, it can't because they are insulated
- Sun Feb 09, 2020 10:14 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Strong Acids
- Replies: 6
- Views: 320
Re: Strong Acids
It's also helpful to know that the problem will most likely give you the Ka and Kb of weak acids and bases, so if they're not given, the acid/base is most likely strong.
- Sun Feb 09, 2020 7:16 pm
- Forum: Thermodynamic Definitions (isochoric/isometric, isothermal, isobaric)
- Topic: Clarification on a group of energy concepts
- Replies: 3
- Views: 156
Re: Clarification on a group of energy concepts
I don't think we need to know rotational, vibrational, and transitional energy. We never went over those in class from what I remember. For q at constant pressure and constant volume, the difference is in the equation. When a rxn is under constant pressure, the equation is q = nCp\Delta T . Under co...
- Sun Feb 09, 2020 12:11 am
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Dashed line
- Replies: 2
- Views: 86
Re: Dashed line
Yeah, it just indicates that it is the bond enthalpy for resonance bonds, so the value should be greater than a single bond but lower than a double bond.
- Thu Feb 06, 2020 5:48 pm
- Forum: Calculating Work of Expansion
- Topic: Kelvin vs Celsius
- Replies: 5
- Views: 176
Re: Kelvin vs Celsius
It should be safe to always use Kelvin, since it is always the unit used in formulas. The temperature is sometimes given in Celsius, so you have to convert it, which is just adding 273.15
- Tue Feb 04, 2020 9:00 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: work/energy units
- Replies: 3
- Views: 151
Re: work/energy units
Yeah, both are okay. Usually, you would just match the units that the problem gives or use J and scientific notation if the answer is large.
- Sun Feb 02, 2020 11:39 pm
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Test 1 Pressure
- Replies: 5
- Views: 292
Re: Test 1 Pressure
An increase in pressure will cause the reaction to shift towards the side with less moles. A decrease in pressure will cause the reaction to shift towards the side with more moles. This is because, in equilibrium, the reaction is trying to stay balanced. If the pressure changes, the system is trying...
- Sun Feb 02, 2020 1:51 pm
- Forum: Ideal Gases
- Topic: pv=nrt
- Replies: 9
- Views: 396
Re: pv=nrt
How does a change in pressure affect the other variables: v, n, r, and t? It's easier if you just look at the equation PV = nRT. If pressure changes, volume changes in the opposite direction because it is multiplied with P and it needs to make up for the change. However, n and T change in the same ...
- Sun Feb 02, 2020 1:47 pm
- Forum: Ideal Gases
- Topic: pv=nrt
- Replies: 9
- Views: 396
Re: pv=nrt
How does a change in pressure affect the other variables: v, n, r, and t? A change in pressure can be caused by a change in V, n, or T. R is never changed. An increase in pressure could be caused by: a decrease in volume, an increase in number of moles, or an increase in temperature. A decrease in ...
- Mon Jan 27, 2020 7:19 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Calorimeter
- Replies: 7
- Views: 527
Re: Calorimeter
A calorimeter is just the tool used to measure specific heat. I doubt we'll really need to know the parts and how it works, but the important part to remember is which variable is constant: pressure or volume. I think this is because the equation or the units we have to use change depending on which...
- Sun Jan 26, 2020 11:18 am
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Spontaneous Question
- Replies: 3
- Views: 188
Re: Spontaneous Question
What is a spontaneous reaction and when did we start learning about this in class?
- Sun Jan 26, 2020 11:11 am
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Negative Square Root solving an ICE box
- Replies: 13
- Views: 584
Re: Negative Square Root solving an ICE box
Usually the math is pretty straightforward in the chemistry problems we get, so if you got a negative number inside the square root, then you probably did something wrong.
- Fri Jan 24, 2020 5:17 pm
- Forum: Ideal Gases
- Topic: Homework 3
- Replies: 6
- Views: 481
Re: Homework 3
Yeah, it should be fine. I did homework problems from Acids and Bases for week 3.
- Fri Jan 24, 2020 5:12 pm
- Forum: Ideal Gases
- Topic: Inert Gas
- Replies: 12
- Views: 705
Re: Inert Gas
Yeah, noble and inert gases are both terms used to describe the last column of the periodic table
- Fri Jan 24, 2020 4:42 pm
- Forum: Phase Changes & Related Calculations
- Topic: Steam vs. Boiling Water
- Replies: 10
- Views: 508
Re: Steam vs. Boiling Water
Despite this energy difference, though, the liquid and gas are at the same temperature? How does that work? The liquid and gas aren't at the same temperature. The horizontal lines on the graph represent phase changes. They are flat because the heat is being used to break bonds, which absorbs the en...
- Thu Jan 23, 2020 5:28 pm
- Forum: Non-Equilibrium Conditions & The Reaction Quotient
- Topic: pKa/Pkb vs pH/pOH
- Replies: 3
- Views: 162
Re: pKa/Pkb vs pH/pOH
I think the only similarity between them is the p that's before all of them. The p is just showing that it is the -log value of whatever is next to it. Ka and Kb are the acidity and basicity constants, aka acid and base dissociation constants. H and OH is just the concentrations of hydronium [H3O+] ...
- Thu Jan 23, 2020 5:11 pm
- Forum: Non-Equilibrium Conditions & The Reaction Quotient
- Topic: pKa/pKb and Ka/Kb
- Replies: 5
- Views: 183
Re: pKa/pKb and Ka/Kb
The p before Ka and Kb just shows that it is the -log value of Ka and Kb, like how pH and pOH means -log[H3O+] and -log[OH-]
- Sun Jan 12, 2020 12:07 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Quadratic Equation
- Replies: 3
- Views: 125
Re: Quadratic Equation
A, B, and C are the coefficients in the quadratic equation Ax^{2}+Bx+C=0 These values are then plugged into the quadratic formula x=-B\pm \frac{\sqrt{B^{2}-4AC}}{2A} The quadratic equation is what we might get after trying to solve for x after setting up an ICE box and plugging the values into the e...
- Sat Jan 11, 2020 11:48 pm
- Forum: Non-Equilibrium Conditions & The Reaction Quotient
- Topic: Cancelling out the X
- Replies: 3
- Views: 261
Re: Cancelling out the X
The whole point of the quadratic equation is to solve for x.
After making the equation equal to 0, if it looks like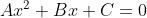 , then you can plug the coefficients into the formula
, then you can plug the coefficients into the formula 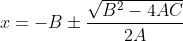 and find the value of x.
and find the value of x.
After making the equation equal to 0, if it looks like
- Sat Jan 11, 2020 6:16 pm
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Change in pressure
- Replies: 5
- Views: 270
Re: Change in pressure
The equilibrium will shift to whichever side has less moles. That way, the molecules take up less space and the pressure is minimized.
- Sat Jan 11, 2020 4:10 pm
- Forum: Ideal Gases
- Topic: converting Kc to Kp
- Replies: 13
- Views: 450
Re: converting Kc to Kp
Naren_Ramesh_4F wrote:When asked the equilibrium constant of a chemical reaction involving gases, do we solve for Kc or Kp?
If the reaction involves gases, we will most likely be solving for Kp unless the question specifically asks us for Kc, in which case we will need to convert from Kp to Kc.
- Sat Jan 11, 2020 3:59 pm
- Forum: Ideal Gases
- Topic: Solving for K when only given balanced equation [ENDORSED]
- Replies: 6
- Views: 266
Re: Solving for K when only given balanced equation [ENDORSED]
P is used to indicate that the reactants and products are gases. You will know to use P if every molecule has a (g) after them, such as Cl2(g). If you were asked to solve K and weren't given any values, the question was probably just asking for the expression. Then, you would use the brackets [] for...
- Tue Nov 12, 2019 2:04 pm
- Forum: Administrative Questions and Class Announcements
- Topic: Week 7 Homework
- Replies: 7
- Views: 298
Re: Week 7 Homework
We haven't moved past Chemical Bonds yet, so I think doing problems from there would be safest.
- Wed Nov 06, 2019 1:13 pm
- Forum: Bond Lengths & Energies
- Topic: Midterm
- Replies: 8
- Views: 476
Re: Midterm
Will bond lengths and energies be on the midterm? What is the topic that the midterm will cover up to?
- Mon Nov 04, 2019 1:06 am
- Forum: Electronegativity
- Topic: Comparing electronegativities
- Replies: 2
- Views: 130
Re: Comparing electronegativities
I learned in my discussion that when determining electronegativity, the elements along the same row/period tend to have a higher electronegativity than those that are the same distance away in the column/group. I think this is because of the radii of the atoms. There is not as much of a difference a...
- Mon Nov 04, 2019 12:50 am
- Forum: Octet Exceptions
- Topic: Uhhh
- Replies: 3
- Views: 161
Re: Uhhh
Other exceptions include radicals and Lewis acids and bases. Radicals are species (atoms, ions, or molecules) with unpaired electrons. Lewis acids and bases are species that have donated/accepted electron pairs.
- Mon Nov 04, 2019 12:38 am
- Forum: Octet Exceptions
- Topic: Expanded Valence Shells
- Replies: 4
- Views: 172
Re: Expanded Valence Shells
Lauren Sanchez 3D wrote:Is this why some elements end up breaking the octet rule?
Yeah, elements in period 3 or higher don't have to abide by the octet rule.
- Sun Oct 27, 2019 4:02 pm
- Forum: Electron Configurations for Multi-Electron Atoms
- Topic: Pauli Exclusion Principle
- Replies: 3
- Views: 217
Re: Pauli Exclusion Principle
I don't think we really need to know why an orbital can have up to 2 electrons, simply that it does.
- Sun Oct 27, 2019 1:46 pm
- Forum: Electron Configurations for Multi-Electron Atoms
- Topic: Electron Configuration
- Replies: 3
- Views: 246
Re: Electron Configuration
Vanadium's electron configuration would be written [Ar]3d3, 4s2. Remember 3d has lower energy than 4s, so it is written first. You would only take electrons from the s orbital if it's to complete a half-full or full d subshell, as in the instance of Cr:[Ar]3d5,4s1. A good way to remember this is to...
- Sun Oct 27, 2019 1:41 pm
- Forum: Electron Configurations for Multi-Electron Atoms
- Topic: Same spin
- Replies: 10
- Views: 558
Re: Same spin
When would you see them have parallel spins? If they are more stable with different spins, what would constitute them having the same spin? Elements such as carbon and nitrogen have parallel spins in the 2p subshell because it has multiple orbitals and electrons with parallel spins typically occupy...
- Mon Oct 21, 2019 12:16 am
- Forum: Properties of Light
- Topic: Wave Properties of Sound
- Replies: 4
- Views: 222
Re: Wave Properties of Sound
An example of constructive interference would be like two different speakers in the same area playing the exact same music at the exact same time. They are emitting separate waves, which are then combining to create a louder sound.
- Mon Oct 21, 2019 12:11 am
- Forum: Properties of Light
- Topic: Relationships
- Replies: 3
- Views: 169
Re: Relationships
Photons are, according to the textbook glossary, "a particle-like packet of electromagnetic radiation." To make it easier, think of them like packets of energy. Energy can be anything from visible light to heat (which is infrared radiation) to things we can't even sense, like x-rays. So li...
- Sun Oct 20, 2019 11:53 pm
- Forum: Properties of Light
- Topic: Variables and what they mean
- Replies: 9
- Views: 508
Re: Variables and what they mean
Be careful about mixing up the variables for frequency and velocity! The frequency variable is more curved than the regular v used for velocity.
- Sun Oct 20, 2019 11:24 pm
- Forum: Properties of Light
- Topic: Midterm Exam
- Replies: 21
- Views: 680
Re: Midterm Exam
The test and exam schedule on the chem website shows that the midterm is gonna be on Wednesday, November 6, which is in Week 6, from 6-8 pm.
- Sun Oct 13, 2019 11:59 pm
- Forum: DeBroglie Equation
- Topic: Constructive vs. Destructive Interference
- Replies: 5
- Views: 193
Re: Constructive vs. Destructive Interference
With constructive interference, is the resulting amplitude just the lengths of the individual waves added together? Or can it not be measured that way? I think it does work like that. The book doesn't say anything, so I don't think we need to know it for chem, but I remember learning that in AP Phy...
- Sun Oct 13, 2019 11:56 pm
- Forum: DeBroglie Equation
- Topic: Constructive vs. Destructive Interference
- Replies: 5
- Views: 193
Re: Constructive vs. Destructive Interference
In constructive interference, the peaks and troughs (highest and lowest parts) of a wave align and the amplitude of the combined wave increases (it gets taller). In destructive interference, the peaks of one wave align with the troughs of the other and vice versa and the resulting wave's amplitude i...
- Sun Oct 13, 2019 11:33 pm
- Forum: Ideal Gases
- Topic: Reading the textbook
- Replies: 262
- Views: 149993
Re: Reading the textbook
What do you guys recommend when reading the textbook? Because it can get really confusing and I don't understand what to take notes on. Also, should we read them before class and take notes or after lecture? I think we can read the textbook whenever we want. If you prefer to learn the topics on you...
- Sun Oct 13, 2019 11:21 pm
- Forum: Ideal Gases
- Topic: Reading the textbook
- Replies: 262
- Views: 149993
Re: Reading the textbook
When reading from the textbook, what should you take notes on? Or rather what should you focus on? It's different for everyone. I usually focus on the bolded words and formulas since those are usually what we have to know. However, if I don't understand something, I read the paragraphs that cover t...
- Sun Oct 13, 2019 11:11 pm
- Forum: Properties of Electrons
- Topic: Photons
- Replies: 5
- Views: 337
Re: Photons
I don't see how photons can be negative because they are packets of energy. However, the change in energy is negative if the amount of energy decreases. A positive energy change would result in an increase in energy. I don't know if the energy of a photon can change though, since it is usually the r...
- Sat Oct 12, 2019 5:25 pm
- Forum: Accuracy, Precision, Mole, Other Definitions
- Topic: Test 1 [ENDORSED]
- Replies: 107
- Views: 20484
Re: Test 1 [ENDORSED]
If we need to use the answer of one question for the next question and our answer for the first question is wrong, will we not get full credit for the second, even if our calculations on the second were correct?
- Mon Oct 07, 2019 12:34 am
- Forum: Administrative Questions and Class Announcements
- Topic: powers of 10 in answers
- Replies: 2
- Views: 122
Re: powers of 10 in answers
Scientific notation usually just helps shorten answers that are really long, like 10^9 or 10^-9, especially when you need to use a specific measurement, like grams instead of kilograms. Also, if the answer is something like 5,000 g and you need 2 sig figs for the answer, you would use scientific not...
- Mon Oct 07, 2019 12:23 am
- Forum: Accuracy, Precision, Mole, Other Definitions
- Topic: sig figs
- Replies: 20
- Views: 974
Re: sig figs
This is what I got from Appendix 1 (1C) in the textbook: -Addition and Subtraction: round to the lowest number of sig figs after the decimal -Multiplication and Division: round to the lowest number of sig figs in general -Zeros between nonzero numbers (ex. 30.1): significant -Zeros after the decimal...
- Mon Oct 07, 2019 12:05 am
- Forum: Accuracy, Precision, Mole, Other Definitions
- Topic: Showing work in detail
- Replies: 7
- Views: 634
Re: Showing work in detail
I think you can just do what's comfortable for you. As long as you show some work that let's the TAs understand your train of thought and get the right answer, you should get full credit.
- Sun Oct 06, 2019 11:53 pm
- Forum: Accuracy, Precision, Mole, Other Definitions
- Topic: avogadro's number
- Replies: 4
- Views: 319
Re: avogadro's number
Basically, Avogadro's number let's you convert between moles and molecules/atoms/formula units.
When converting from moles, multiply by Avogadro's number. When converting from molecules/atoms/formula units, divide.
When converting from moles, multiply by Avogadro's number. When converting from molecules/atoms/formula units, divide.
- Sun Oct 06, 2019 11:41 pm
- Forum: Accuracy, Precision, Mole, Other Definitions
- Topic: Rounding
- Replies: 12
- Views: 871
Re: Rounding
This is what I found in Appendix 1 (1C) of the textbook: For addition and subtraction, you round to the lowest number of digits after the decimal. For multiplication and division, you round to the smallest number of sig figs in general. If there is a 0 right after the decimal and nothing after, like...

