I think catalysts can be included sometimes. See this post by Chem_Mod: viewtopic.php?t=1727
Hope this helps!
Search found 100 matches
- Tue Mar 10, 2020 3:22 pm
- Forum: Arrhenius Equation, Activation Energies, Catalysts
- Topic: Catalysts and Rate Law
- Replies: 4
- Views: 326
- Tue Mar 10, 2020 3:20 pm
- Forum: Reaction Mechanisms, Reaction Profiles
- Topic: pre equilibrium approach
- Replies: 1
- Views: 169
Re: pre equilibrium approach
Usually when you are given a mechanism, there are multiple reactions that are fast and slow. The most important step would be the slow step because the slowest step determines the rate. Since there may be a fast reaction before a slow step, this means that the product of the fast step, which is also...
- Tue Mar 10, 2020 3:17 pm
- Forum: Administrative Questions and Class Announcements
- Topic: 14B acids and bases section
- Replies: 2
- Views: 229
Re: 14B acids and bases section
We did go over this in 14A, but I think you can also think though this using your knowledge of equilibrium. If you get rid of the log for pH and pKa, then you will be left with a concentration of hydronium ions and the Ka. When the pH is lower than pKa, this means that there are more hydronium ions ...
- Tue Mar 10, 2020 3:13 pm
- Forum: Environment, Ozone, CFCs
- Topic: NO3
- Replies: 6
- Views: 894
Re: NO3
I'm not sure what the context for this question is, but usually something would not be included in the overall equation if it is a catalyst or an intermediate. Hope this helps!
- Tue Mar 10, 2020 3:10 pm
- Forum: First Order Reactions
- Topic: determining Kr
- Replies: 5
- Views: 379
Re: determining Kr
Yes, you can find k by calculating the slope. Since it is linear, you can use rise over run. If you look at the examples in the textbook, they use two points on the line to calculate the slope. Hope this helps!
- Tue Mar 03, 2020 3:59 pm
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: 6.53
- Replies: 2
- Views: 228
Re: 6.53
In order to reach equilibrium, you would want the half cell with the greater concentration to go down in concentration and the half cell with the smaller concentration to go up in concentration. To do this, the ions in the high concentration half cell need to be reduced to a solid so that the ions a...
- Tue Mar 03, 2020 3:58 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: 6L7c
- Replies: 2
- Views: 283
Re: 6L7c
In the reaction, there are hydroxide ions, but since everything else is shown as a solid, the hydroxide ions are written as KOH to show that the hydroxide ions are bounded to something and are not simply by themselves. Hope this helps!
- Tue Mar 03, 2020 3:56 pm
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: 6N. 15
- Replies: 1
- Views: 161
Re: 6N. 15
I think this is because the nickel ions are reacting with they hydroxide ions, which causes less nickel ions to be in the solution since they will be forming Ni(OH)2 instead. Hope this helps!
- Tue Mar 03, 2020 3:54 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: 6L.7 part b Homework Help
- Replies: 2
- Views: 268
Re: 6L.7 part b Homework Help
It is implied that water is involves when you put that the ions are (aq), so you don't need to include water again because that would make it redundant. Hope this helps!
- Tue Mar 03, 2020 3:53 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: 6M.5 Part b
- Replies: 2
- Views: 247
Re: 6M.5 Part b
In order to match the given equation, then bromine must be involved in the oxidation half reaction. Hence, you get 0.92 - 1.09, which is negative and indicates that the reaction is non-spontaneous.
- Wed Feb 26, 2020 12:39 pm
- Forum: Balancing Redox Reactions
- Topic: 6L.9
- Replies: 2
- Views: 232
Re: 6L.9
I think they were mainly excluded because they're spectator ions. I don't think including or excluding them will affect your answer since they are neither oxidized or reduced, so if you included them, your answer is probably fine. Hope this helps!
- Wed Feb 26, 2020 12:37 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: 6L.7
- Replies: 3
- Views: 305
Re: 6L.7
I think they just used nickel because it's a nickel-cadmium cell, so I assumed that those would be the electrodes. However, the sole purpose is to use an inert metal, so I think that using platinum or graphite would also be alright. If you want to double check, maybe try asking Professor Lavelle or ...
- Wed Feb 26, 2020 12:31 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: 6L.5
- Replies: 2
- Views: 231
Re: 6L.5
For part C, you can split HCl into a positive hydrogen ion and negative chlorine ion. This will give you the two half reactions. For part D, Au is being oxidized to produce the 3+ ion and reduced to produce a solid. Since there is simply a transfer of electrons between two Au atoms, Au is both the o...
- Wed Feb 26, 2020 12:26 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: 6N.9
- Replies: 2
- Views: 218
Re: 6N.9
I believe you are supposed to determine this based on their reduction potentials. If you look at the table of reduction potentials, the reduction of tin has a lower potential than hydrogen, which means that tin is less likely to be reduced than hydrogen. Based on this information, you can infer that...
- Wed Feb 26, 2020 12:23 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Standard Cell Potential
- Replies: 2
- Views: 216
Re: Standard Cell Potential
I'm not sure about the specifics of 6M.2 since I did not attempt that problem, but in general you cannot just add standard potentials of reactions because you are usually given the standard reduction potential, which is the potential that stems from a reduction reaction. However, in a redox reaction...
- Tue Feb 18, 2020 3:47 pm
- Forum: Balancing Redox Reactions
- Topic: Odd number of electrons
- Replies: 3
- Views: 357
Re: Odd number of electrons
I think that this has to do with conservation of mass. Mass can neither be created nor destroyed, which is why the number of electrons given off must be gained by another atom. For the purposes of this class, the number of electrons transferred on both sides should be equal. Hope this helps!
- Tue Feb 18, 2020 3:44 pm
- Forum: Van't Hoff Equation
- Topic: Units of Partial Pressure in 5G-13, 5G-15
- Replies: 3
- Views: 278
Re: Units of Partial Pressure in 5G-13, 5G-15
As stated before, bars are approximately equal to atmospheres, but in general this shouldn't matter as long as they are all in the same units. When you are calculating Q, you usually use the values without units because the partial pressures are representing the activities of each compound, which ha...
- Tue Feb 18, 2020 3:42 pm
- Forum: Balancing Redox Reactions
- Topic: Reducing/Oxidizing Agent
- Replies: 4
- Views: 289
Re: Reducing/Oxidizing Agent
You can use mnemonic devices to help you keep oxidation and reduction straight. For instance, OIL RIG - oxidation is loss, reduction is gain. Something that is oxidized will lose electrons while something that is reduced will gain electrons. An oxidizing agent or reducing agent is what causes oxidat...
- Tue Feb 18, 2020 3:40 pm
- Forum: Balancing Redox Reactions
- Topic: Oxidation Numbers
- Replies: 2
- Views: 161
Re: Oxidation Numbers
Although there are some oxidation numbers that you might have to memorize, you can determine them from the position of elements on the periodic table and the other elements they are bonded with. For instance, oxygen is in the second to last column in the periodic table, which means that it has 6 val...
- Tue Feb 18, 2020 3:38 pm
- Forum: Balancing Redox Reactions
- Topic: Half Reactions
- Replies: 7
- Views: 486
Re: Half Reactions
We probably won't be given anything that needs to be split up into anything more than half reactions. After all, in redox reactions, something is oxidized and something is reduced, so one half reaction shows the oxidation and one half reaction shows the reduction. Hope this helps!
- Sat Feb 15, 2020 2:24 pm
- Forum: Third Law of Thermodynamics (For a Unique Ground State (W=1): S -> 0 as T -> 0) and Calculations Using Boltzmann Equation for Entropy
- Topic: Boltzmann Equation
- Replies: 10
- Views: 610
Re: Boltzmann Equation
The Boltzmann equation calculates residual entropy, or in other words, the entropy of molecules purely based on their position/arrangement since we are assuming that the temperature is 0 K.
- Sat Feb 15, 2020 2:22 pm
- Forum: Calculating Work of Expansion
- Topic: Isothermal Irreversible
- Replies: 6
- Views: 1424
Re: Isothermal Irreversible
Since both cases are isothermal, you can assume that delta U is 0. Work is calculated differently in these two scenarios because during an irreversible expansion, there is a large difference between the internal pressure and the external pressure, so expansion occurs quickly. This is why we can use ...
- Sat Feb 15, 2020 2:15 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: Delta U = 0
- Replies: 8
- Views: 556
Re: Delta U = 0
Another way you could think of this is using the equation 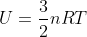 that is on our equation sheet. You can also write this equation as
that is on our equation sheet. You can also write this equation as 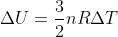 , so if an environment is isothermal (meaning that delta T is 0), then delta U would also be 0. Hope this helps!
, so if an environment is isothermal (meaning that delta T is 0), then delta U would also be 0. Hope this helps!
- Sat Feb 15, 2020 2:12 pm
- Forum: Entropy Changes Due to Changes in Volume and Temperature
- Topic: Rules for constant pressure
- Replies: 4
- Views: 281
Re: Rules for constant pressure
As stated before, in constant pressure, you can assume that \Delta H = q_{}p and w = -P_{ext}\Delta V while in constant volume, you can assume that w = 0 since delta V would be 0. However, you can also use the heat capacity ( C_{p}=\frac{5}{2}R ) for constant pressure and the heat capacity ( C_{v}=\...
- Sat Feb 15, 2020 2:08 pm
- Forum: Van't Hoff Equation
- Topic: Constant
- Replies: 3
- Views: 170
Re: Constant
The R constant depends on the units you are given and/or the units you want to end up with. For instance, if you are given volume in liters and pressure in atm, then use the constant with those units in it (8.206x10^-2). If you are calculating entropy, use the 8.314 R constant since you want entropy...
- Tue Feb 04, 2020 10:18 pm
- Forum: Third Law of Thermodynamics (For a Unique Ground State (W=1): S -> 0 as T -> 0) and Calculations Using Boltzmann Equation for Entropy
- Topic: Calculating W
- Replies: 3
- Views: 186
Re: Calculating W
This is just saying that multiplying Avogadro's number with the Boltzmann's constant will equal the constant R. If you look on your constants and equations sheet, there is a constant R listed at the top, which is the constant we use in equations like PV = nRT. Hope this helps!
- Tue Feb 04, 2020 10:13 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Bomb Calorimeters
- Replies: 2
- Views: 162
Re: Bomb Calorimeters
I think all we need to know about bomb calorimeters is that they have constant volume. Whenever a problem says a reaction takes place in a bomb calorimeter, you can assume that there is no work since there is no change in volume. Hope this helps!
- Tue Feb 04, 2020 10:12 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: textbook problem 4D.15
- Replies: 2
- Views: 156
Re: textbook problem 4D.15
In this problem, they give you the enthalpy for the combustion of three different compounds. In combustion, you know that the compound will react with oxygen to produce carbon dioxide and water vapor. Once you balance them, this is how you get the three equations. From there, you can use Hess's Law ...
- Tue Feb 04, 2020 10:10 pm
- Forum: Calculating Work of Expansion
- Topic: When to use different R values
- Replies: 2
- Views: 122
Re: When to use different R values
You can determine what R value to use based on the units. Compare the units you are given to the units of each constant and go from there. Hope this helps!
- Tue Feb 04, 2020 10:09 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: standard reaction enthalpy
- Replies: 3
- Views: 191
Re: standard reaction enthalpy
If it is standard reaction enthalpy, it may say kJ or kJ/mol, but it is implied that the standard reaction enthalpy given is for one "mole" of reaction. You can't really have a mole of a reaction, so this just means that the given standard reaction enthalpy is the enthalpy proportional to ...
- Tue Jan 28, 2020 10:51 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: 4E.5
- Replies: 2
- Views: 128
Re: 4E.5
Another way you can think of this instead of trying to figure out which bonds are forming and which bonds are breaking is to break all the bonds in the reactants and to form all the bonds in the product. In this problem, you would be breaking 4 C-H bonds and 4 C-Cl bonds and then forming 4 C-H bonds...
- Tue Jan 28, 2020 10:47 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: 4d 15
- Replies: 2
- Views: 108
Re: 4d 15
I'm not sure if this is correct, but I approached the question a little differently from using Hess's Law. The question gives the enthalpies of combustion for each compound. In other words, these are the enthalpies required to break these compounds down. In the overall equation, C_{2}H_{2} and H_{2}...
- Tue Jan 28, 2020 10:41 pm
- Forum: Phase Changes & Related Calculations
- Topic: Phase change and temp
- Replies: 8
- Views: 309
Re: Phase change and temp
The temperature remains constant during a phase change because the energy is used to break bonds to change the phase rather than to change the temperature. Hope this helps!
- Tue Jan 28, 2020 10:40 pm
- Forum: Phase Changes & Related Calculations
- Topic: Units
- Replies: 16
- Views: 870
Re: Units
Kelvin is just the degrees Celsius plus 273.15. However, one degree change in Kelvin is equal to one degree change in Celsius. For this reason, I think we can interchange them since we are mainly focused on the change in temperature rather than what the temperature actually is. Hope this helps!
- Tue Jan 28, 2020 10:38 pm
- Forum: Calculating pH or pOH for Strong & Weak Acids & Bases
- Topic: 6D.9 - Calculating pH and Ka for benzoic acid
- Replies: 1
- Views: 761
Re: 6D.9 - Calculating pH and Ka for benzoic acid
I don't think the way I solved this was the same as the book, but this is the way I solved it: in this problem, benzoic acid reacts with water to produce its conjugate base and hydronium ions. You can draw out an ICE table for this. At equilibrium, the concentration of the conjugate base and hydroni...
- Wed Jan 22, 2020 7:51 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: K vs Kc
- Replies: 2
- Views: 93
Re: K vs Kc
Adding on to previous replies, you should use the Kc column for most problems in the textbook since they involve concentrations. I used the Kc column when I did the homework and it worked out well. However, on an actual test, you would be given the correct K value.
- Wed Jan 22, 2020 7:49 pm
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Adding Heat and Constant Temperature
- Replies: 2
- Views: 122
Re: Adding Heat and Constant Temperature
Adding on to previous responses, the temperature is measuring a much larger outside system compared to the small system your reaction may be taking place in. Hence, any changes in temperature of the small system may not heavily impact the environment, which may explain why the temperature of the out...
- Wed Jan 22, 2020 7:45 pm
- Forum: Phase Changes & Related Calculations
- Topic: Thermochemistry homework problems
- Replies: 2
- Views: 79
Re: Thermochemistry homework problems
Homework isn't really strict. Usually, you do homework on the sections discussed in class. Since we talked about enthalpy and state properties, I would assume you can just find the section that talks about the same topics and do the problems from there. However, if you go ahead to something we have ...
- Wed Jan 22, 2020 7:43 pm
- Forum: Phase Changes & Related Calculations
- Topic: State Property
- Replies: 6
- Views: 390
Re: State Property
From what I understand, state properties are values that are path independent. For instance, when we ask for the change in a state property, we can find the difference between the final and initial values to calculate the change. Meanwhile, for non-state properties, the path is important. This means...
- Wed Jan 22, 2020 7:40 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Ice Box Question
- Replies: 2
- Views: 113
Re: Ice Box Question
You always subtract some sort of variable from the reactants and add some sort of variable to the products in an ice box. It is only X if all of the reactants and products have a one to one ratio. However, the variables are based on molar ratios, so if a reactant has a one to two ratio to a product,...
- Tue Jan 14, 2020 11:32 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: ICE Chart
- Replies: 5
- Views: 347
Re: ICE Chart
Problems will usually give you the chemical equation in the order they want the ICE table to be organized, but if not, they will usually give you the initial concentrations of one side of a chemical equation. Organize your ICE table so that those initial concentrations are on the left while the mole...
- Tue Jan 14, 2020 11:28 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Equilibrium Constant
- Replies: 4
- Views: 133
Re: Equilibrium Constant
Like other people said, solids are always excluded since they do not have a concentration while pure liquids are always excluded since they do not change concentrations. In terms of aqueous solutions, the solvent is not included, but the solutes are. You can tell the difference because solvents will...
- Tue Jan 14, 2020 11:25 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Kp vs Kc
- Replies: 4
- Views: 131
Re: Kp vs Kc
Like other people said, it depends on what is given in the problem. If they prefer a specific form, it will probably be specified or you will be given either concentrations or partial pressures. However, in Lyndon's workshop, he said that concentration is more commonly used, so you can probably assu...
- Tue Jan 14, 2020 11:23 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Acids and Bases
- Replies: 3
- Views: 127
Re: Acids and Bases
The way I understood this was that when an acid is added to water, it just introduces more hydrogen ions that will bond with water to create hydronium ions and bond with hydroxide ions to produce water. This is why the hydronium ion concentration will increase while the hydroxide ion concentration w...
- Tue Jan 14, 2020 11:19 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Thermodynamically Stable?
- Replies: 3
- Views: 145
Re: Thermodynamically Stable?
When something is more thermodynamically stable, it just means that it is less likely that it will dissociate since stable molecules want to stay the way they are. To figure out part c, where it asks which one is more stable, just look at whether chlorine gas or fluorine gas dissociates less. The on...
- Thu Jan 09, 2020 9:49 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Solvents
- Replies: 5
- Views: 165
Re: Solvents
Another way to think of this is by seeing what happens if you do include the solvent when calculating the equilibrium constant. Since the solvent has a negligible change in concentration, the approximately same value would be included in both the numerator and the denominator, thus causing the conce...
- Thu Jan 09, 2020 9:45 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: 5H.3
- Replies: 4
- Views: 151
Re: 5H.3
If this were a problem on a test, the K values would have to be given to you. The only time you would ever have to calculate K is if you could find the concentrations of all reactants and products or the partial pressures of all reactants and products to plug into the K expression. This is not the c...
- Thu Jan 09, 2020 9:43 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: solids/liquids
- Replies: 4
- Views: 167
Re: solids/liquids
Another way to think about this is that the equilibrium constant is the ratio between the products and the reactants. Since the concentration of a solid (since solids do not have a concentration) and the concentration of a pure liquid/solvent do not change, including them in the ratio would mean tha...
- Thu Jan 09, 2020 9:40 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: units of K
- Replies: 10
- Views: 529
Re: units of K
We went over this in class a little bit, but there is also a section in the textbook that explains this if you would like to reference this in case my explanation does not make sense. Essentially, the equilibrium constant is the ratio between the activity of the products and the activity of the reac...
- Thu Jan 09, 2020 9:36 am
- Forum: Administrative Questions and Class Announcements
- Topic: Homework 1
- Replies: 18
- Views: 676
Re: Homework 1
I believe that the homework is graded for completion, but I am not completely sure. However, you can check your answers in the back of the book or by using the solution manual if you have it. I have been told that the homework is supposed to be for our own benefit, so I suppose the amount of effort ...
- Thu Dec 05, 2019 5:27 pm
- Forum: Calculating pH or pOH for Strong & Weak Acids & Bases
- Topic: finding molarity
- Replies: 4
- Views: 410
Re: finding molarity
Molarity of an acid and base is the same as molarity of any solution, which we learned about in the beginning of the quarter. It is simply the number of moles of solute divided by the total volume of the solution. However, for acids and bases specifically, we need to know how normal molarity relates...
- Thu Dec 05, 2019 5:23 pm
- Forum: Hybridization
- Topic: hybridization
- Replies: 1
- Views: 99
Re: hybridization
Essentially, hybridization is a concept that explains what we experimentally observe about molecules. For instance, carbon usually has four bonds, but when drawing an Aufbau diagram, carbon has two paired electrons and two lone electrons, suggesting that carbon should only be able to have two bonds....
- Thu Dec 05, 2019 5:17 pm
- Forum: Naming
- Topic: sodium bisoxalato(diaqua)ferrate(III) (homework 9C.3D)
- Replies: 2
- Views: 229
Re: sodium bisoxalato(diaqua)ferrate(III) (homework 9C.3D)
1. You use bis, tris, or tetrakis instead of the usual prefixes when the ligand already has a Greek prefix or if it is polydentate. In this case, oxalate is bidentate, so it would be named bisoxalato. 2. Usually ligands are written in alphabetical order (excluding prefixes), but I'm not really sure ...
Re: 9C.1 a
Cyano and cyanido is the same thing. They are just from different naming conventions. Both are acceptable. There is another post about this by Chem_Mod: viewtopic.php?t=2351
- Thu Dec 05, 2019 5:04 pm
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: Determining Coordination Number
- Replies: 2
- Views: 237
Re: Determining Coordination Number
I believe some ligands can form double bonds or triple bonds, but I think that would only count once toward the coordination number.
- Thu Nov 28, 2019 12:52 am
- Forum: Bronsted Acids & Bases
- Topic: Lewis and Bronsted
- Replies: 4
- Views: 286
Re: Lewis and Bronsted
Yes, the main difference between Bronsted acids and bases compared to Lewis acids and bases is merely what they focus on in their definitions. The Lewis acid and base definition is more general since a Lewis acid is simply an electron pair acceptor while a Lewis base is an electron pair donor. The B...
Re: Chelation
I think that we just need to be able to draw a ligand connected to a central metal atom, and if the ligand forms a ring with the metal atom, then it is considered a chelate. This is significant because the shape of a molecule can greatly affect its function. I think the effect of a chelate is that t...
- Thu Nov 28, 2019 12:33 am
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: Coordinate covalent bonds
- Replies: 4
- Views: 224
Re: Coordinate covalent bonds
Covalent bonds involve the sharing of electrons. The number of electrons that are shared are determined by whether there is a single, double, or triple bond between atoms. Ionic bonds involve the transfer of valence electrons, but this can involve any number of electrons depending on what atoms are ...
- Thu Nov 28, 2019 12:28 am
- Forum: Amphoteric Compounds
- Topic: Identifying Amphoteric Compounds
- Replies: 2
- Views: 264
Re: Identifying Amphoteric Compounds
As stated before, amphoteric oxides closely match the diagonal band of metalloids on the periodic table. However, they are not exactly the same, so I think it would be better to memorize the elements commonly found in amphoteric oxides.
- Thu Nov 28, 2019 12:27 am
- Forum: Amphoteric Compounds
- Topic: distinguishing oxides
- Replies: 2
- Views: 164
Re: distinguishing oxides
A good way to approach these problems is to use periodic table trends. Metals tend to be found on the left side of the periodic table (group 1 and 2), and metal oxides tend to react with water to form strong bases. Nonmetals tend to be found on the right side of the periodic table, and nonmetal oxid...
- Wed Nov 20, 2019 2:58 pm
- Forum: Student Social/Study Group
- Topic: Lydon's Week 8 Learning Session
- Replies: 4
- Views: 366
Re: Lydon's Week 8 Learning Session
You can probably look up the different Lewis structures. Here are some of the answers I wrote down: 2. AX_{4}E 3. seesaw 4. cannot be determined; might be able to put slightly <120 degrees or slightly <90 degrees 6. AX_{2}E_{3} 7. linear 8. nonpolar 9. 180 degrees 11. AX_{4} 12. tetrahedral 13. pola...
- Wed Nov 20, 2019 2:48 pm
- Forum: Sigma & Pi Bonds
- Topic: Resonance and Sigma/Pi Bonds
- Replies: 3
- Views: 671
Re: Resonance and Sigma/Pi Bonds
I think there was an example of this in the textbook problems. If I understood it correctly, you would just classify sigma and pi bonds for each drawn resonance structure. In other words, I think you would just write all the possibilities. Hope this helps!
- Wed Nov 20, 2019 2:46 pm
- Forum: Shape, Structure, Coordination Number, Ligands
- Topic: Compound Arrangement
- Replies: 2
- Views: 138
Re: Compound Arrangement
Essentially, you just want to make it clear which atoms are bonded. In the class example, the nitrogen is bonded to the nickel, not the hydrogens. This means you would want to draw the bond between nickel and nitrogen. If ammonia is on the left side of the molecule and you write it the normal way (N...
- Wed Nov 20, 2019 2:43 pm
- Forum: Dipole Moments
- Topic: lone pairs and polarity
- Replies: 2
- Views: 244
Re: lone pairs and polarity
Lone pairs of electrons have a negative charge, so I would assume that they affect the polarity of the molecule by producing a partial negative. I'm not sure if this means they have their own dipole moment, but if you were to draw an arrow for it, the reason why the arrow would point toward the lone...
- Wed Nov 20, 2019 2:33 pm
- Forum: Dipole Moments
- Topic: Identifying Induced-Dipole
- Replies: 3
- Views: 236
Re: Identifying Induced-Dipole
I'm not sure if this is the answer you're looking for, but all molecules have induced dipole-induced dipole interactions between them as a result of moving electrons. However, some molecules also have dipole-dipole interactions between them. You can see this when the molecule has polar bonds. When a...
- Thu Nov 14, 2019 2:19 pm
- Forum: Interionic and Intermolecular Forces (Ion-Ion, Ion-Dipole, Dipole-Dipole, Dipole-Induced Dipole, Dispersion/Induced Dipole-Induced Dipole/London Forces, Hydrogen Bonding)
- Topic: H-bonds
- Replies: 4
- Views: 247
Re: H-bonds
As stated before, hydrogen bonds do have dipole moments due to the difference in electronegativity between hydrogen and electronegative atoms like nitrogen, oxygen, and fluorine. This also makes sense because molecules can involve multiple intermolecular forces. In a molecule with hydrogen bonds, di...
- Thu Nov 14, 2019 2:14 pm
- Forum: Bond Lengths & Energies
- Topic: Quiz for Next week dashes/wedges
- Replies: 11
- Views: 654
Re: Quiz for Next week dashes/wedges
I do not think we need to know about dashes and wedges for any tests. I believe we were simply introduced to it so that if it ever came up in the textbook, we would understand what is going on.
- Thu Nov 14, 2019 2:13 pm
- Forum: Interionic and Intermolecular Forces (Ion-Ion, Ion-Dipole, Dipole-Dipole, Dipole-Induced Dipole, Dispersion/Induced Dipole-Induced Dipole/London Forces, Hydrogen Bonding)
- Topic: Interaction potential energy
- Replies: 3
- Views: 156
Re: Interaction potential energy
Since tests can include conceptual topics, it might be a good idea to understand the meaning of the interaction potential energy equation. The negative basically indicates that electrons will naturally take on a favorable position, and the r^6 in the denominator shows how important distance is in in...
- Thu Nov 14, 2019 2:12 pm
- Forum: Bond Lengths & Energies
- Topic: Electron Density
- Replies: 10
- Views: 657
Re: Electron Density
Yes, I believe that lone pairs of electrons are considered regions of electron density since they influence the molecular shape. Please see the attached image.
- Thu Nov 14, 2019 2:08 pm
- Forum: Electronegativity
- Topic: H2O and e- density
- Replies: 2
- Views: 177
Re: H2O and e- density
Lone pairs also count as a region of electron density, which is why water takes on a bent shape.
- Wed Nov 06, 2019 10:56 pm
- Forum: Resonance Structures
- Topic: Formal Charge Question
- Replies: 16
- Views: 938
Re: Formal Charge Question
I believe that the textbook said a resonance structure is any structure in which the atoms are in the same place, but the bonds or location of electrons can move around. In this sense, resonance structures do not all have to be the lowest charge, but I think if we were asked about resonance structur...
- Wed Nov 06, 2019 10:50 pm
- Forum: Dipole Moments
- Topic: Ionic bonds
- Replies: 5
- Views: 309
Re: Ionic bonds
Yes, dipole moments can occur in ions. A dipole moment occurs when there is a difference in electronegativity, and greater differences produce larger dipole moments. Since there is a difference in electronegativity between ions, then there is a dipole moment.
- Wed Nov 06, 2019 10:14 pm
- Forum: Dipole Moments
- Topic: Dipole Moments Determined by Electronegativity?
- Replies: 3
- Views: 226
Re: Dipole Moments Determined by Electronegativity?
Electronegativity refers to how likely it is for an atom to gain another electron. If an atom has a high electronegativity, it is more likely to pull electrons toward itself, so the more electronegative atom will likely have a partial negative charge.
- Wed Nov 06, 2019 10:13 pm
- Forum: Dipole Moments
- Topic: Dipole Moment Changing
- Replies: 3
- Views: 138
Re: Dipole Moment Changing
I would think that it would be the same since the dipole moment is based on how electrons are shared. In a molecule, the atoms and arrangement stays the same, so the pull on the electrons, and thus the dipole moment, would probably be the same.
- Wed Nov 06, 2019 10:11 pm
- Forum: Electronegativity
- Topic: Electron affinity
- Replies: 4
- Views: 286
Re: Electron affinity
Electron affinity refers to the energy released when an atom gains an electron. This may come into play later, but for now, it is important to note that electron affinity is experimentally measured and combined with ionization energy to calculate electronegativity. Hope this helps!
- Sat Nov 02, 2019 10:33 am
- Forum: Bohr Frequency Condition, H-Atom , Atomic Spectroscopy
- Topic: manipulating equations
- Replies: 3
- Views: 141
Re: manipulating equations
Like other people said, I do not think it is necessary to show all the steps, but I do think you should put the original equation and then the final manipulation of it to get all points. For instance, when writing the equation involving Heisenberg's uncertainty principle, I think you have to show th...
- Sat Nov 02, 2019 10:24 am
- Forum: Formal Charge and Oxidation Numbers
- Topic: Minimizing Formal Charges
- Replies: 5
- Views: 424
Re: Minimizing Formal Charges
It depends on the situation. The sum of the formal charges should add up to the overall charge of the atom. If the atom is neutral, then having 2 charges equal to 0, 1 charge equal to +1, and one charge equal to -1 would be optimal. However, if the atom has a specified charge, then the formal charge...
- Sat Nov 02, 2019 10:18 am
- Forum: Resonance Structures
- Topic: Tips for drawing resonance structures
- Replies: 3
- Views: 148
Re: Tips for drawing resonance structures
When I get a problem involving resonance structures, I first treat it like a normal Lewis structure question. First I include all valence electrons and reduce formal charges as much as possible by rearranging the electrons. One thing that I think about afterwards is if it's possible to achieve the s...
- Sat Nov 02, 2019 10:11 am
- Forum: Trends in The Periodic Table
- Topic: Electronegativity trend exception
- Replies: 3
- Views: 2252
Re: Electronegativity trend exception
I believe that the reasoning is similar to why Cr and Cu are exceptions when making their electron configuration. All half filled orbitals has a symmetry that makes it more stable than two half filled orbitals and one filled orbital (in the case of N). This stability causes Nitrogen to be able to ho...
- Sat Nov 02, 2019 10:06 am
- Forum: Electron Configurations for Multi-Electron Atoms
- Topic: Electron configuration rules
- Replies: 1
- Views: 577
Re: Electron configuration rules
The Pauli Exclusion Principle states that each orbital can have two electrons maximum. It also states that if there are two electrons in an orbital, they must have opposite spin. In other words, no two electrons can have the same four quantum numbers. Examples of rule violations would be putting mor...
- Thu Oct 24, 2019 12:33 am
- Forum: Ionic & Covalent Bonds
- Topic: How are lewis structures filled?
- Replies: 6
- Views: 240
Re: How are lewis structures filled?
Yes, the way I think about Lewis structures is like another form of the electron configuration diagrams we draw with arrows. When we draw the arrows in the electron configuration diagrams, we place one arrow in each orbital before filling them in accordance with Hund's Rule. When I draw the electron...
- Thu Oct 24, 2019 12:27 am
- Forum: Electron Configurations for Multi-Electron Atoms
- Topic: coulomb potential energy
- Replies: 4
- Views: 373
Re: coulomb potential energy
I believe that the significance of Coulomb's law is to understand the interaction between electrons and the nucleus by representing it in the form of an equation. For instance, since an electron has a negative charge while the nucleus of an atom has a positive charge, then there is an attractive for...
- Thu Oct 24, 2019 12:20 am
- Forum: Wave Functions and s-, p-, d-, f- Orbitals
- Topic: How do i do 1.D.25?
- Replies: 2
- Views: 137
Re: How do i do 1.D.25?
When I did this problem, I thought that orbital g did not exist, so that's why a 4g orbital cannot exist.
- Thu Oct 24, 2019 12:18 am
- Forum: Ionic & Covalent Bonds
- Topic: What are the exceptions to the octet rule?
- Replies: 5
- Views: 372
Re: What are the exceptions to the octet rule?
Li and Be are also exceptions
- Wed Oct 23, 2019 5:20 pm
- Forum: Electronegativity
- Topic: Electronegativity
- Replies: 14
- Views: 665
Re: Electronegativity
Electronegativity is an element's ability to attract electrons. The reason electronegativity increases as you go across a period from left to right is because the atomic number (or number of protons in the nucleus) increases, thereby giving the element a stronger positive charge to attract negativel...
- Thu Oct 17, 2019 8:45 pm
- Forum: Photoelectric Effect
- Topic: E = pc
- Replies: 3
- Views: 136
Re: E = pc
E=pc is just an equation that relates momentum with energy. We used it in our notes to show how when used in conjunction with E=hv (which relates energy to frequency), we can derive De Broglie's wave equation. I think that we would only use E=pc in a problem if we were given the velocity of an elect...
- Thu Oct 17, 2019 8:26 pm
- Forum: Properties of Light
- Topic: 1A.5
- Replies: 6
- Views: 211
Re: 1A.5
I think that this problem will ultimately have to require at least a little bit of memorization. However, another way I handle these types of problems if I do not remember the specific ranges for each type of wave is that I just remember the order of the types of waves from longest to shortest. Then...
- Thu Oct 17, 2019 8:23 pm
- Forum: Quantum Numbers and The H-Atom
- Topic: quantum number topic
- Replies: 4
- Views: 275
Re: quantum number topic
I'm not sure if you will find this explanation useful, but the way I think about it is that quantum numbers almost act like an ID for electrons. Each electron within an atom has a unique set of quantum numbers. These quantum numbers describe the size, shape, orientation, and spin of each electron. M...
- Thu Oct 17, 2019 8:13 pm
- Forum: Properties of Electrons
- Topic: x,y,z for Electrons
- Replies: 10
- Views: 396
Re: x,y,z for Electrons
Yes, x, y, and z refers to the coordinate plane. In general, I believe that the purpose of having the axes is just so that you can describe the orientation of the electron orbital.
- Thu Oct 17, 2019 4:40 pm
- Forum: Properties of Light
- Topic: 1A7b textbook solution typo?
- Replies: 5
- Views: 202
Re: 1A7b textbook solution typo?
Yes, I believe that this is a typo. I also got 150 pm for this question, and that was the answer that was presented during my TA discussion.
- Wed Oct 09, 2019 12:13 am
- Forum: Limiting Reactant Calculations
- Topic: Theoretical yield
- Replies: 5
- Views: 410
Re: Theoretical yield
Theoretical yield is just the expected mass of product. The question already presents the expected number of moles of product, so the only thing you have to do is convert the expected number of moles to product to the mass of product expected. This can be accomplished by using the molar mass of the ...
- Wed Oct 09, 2019 12:08 am
- Forum: Significant Figures
- Topic: Significant figures on the periodic table
- Replies: 3
- Views: 139
Re: Significant figures on the periodic table
When I talked to Professor Lavelle after the first class, he recommended using all of the figures given in the periodic table provided. I would do this just to be safe and have the most accurate answer possible, but the final decision is up to you. I heard that the first test will be lenient, so you...
- Wed Oct 09, 2019 12:05 am
- Forum: Trends in The Periodic Table
- Topic: Chemistry Community
- Replies: 8
- Views: 500
Re: Chemistry Community
On Saturday, October 5th, Professor Lavelle emailed students saying "Remember Week 1 ends Sunday night so get your 5 points for online discussion," so I think this means that posts on Chemistry Community will continue to be due every Sunday night.
- Wed Oct 09, 2019 12:01 am
- Forum: SI Units, Unit Conversions
- Topic: Problem G.23
- Replies: 3
- Views: 239
Re: Problem G.23
Yes, you do have to add the number of moles of Cl- ions together in order to find the concentration of Cl- ions within the solution. More specifically, this can be accomplished by using dimensional analysis to convert the given masses of NaCl and KCl into the number of moles of Cl- ions. You then ad...
- Tue Oct 08, 2019 11:58 pm
- Forum: Balancing Chemical Reactions
- Topic: Subscripts [ENDORSED]
- Replies: 3
- Views: 221
Re: Subscripts [ENDORSED]
Like Kelsey said, you cannot change the subscript numbers of compounds in the chemical equation because that would make the compounds and reaction completely different. These differences between the textbook and solutions manual are likely typos. For example, L.35 had a typo in the third chemical eq...
- Wed Oct 02, 2019 3:29 pm
- Forum: Limiting Reactant Calculations
- Topic: How do you oxidize a formula?
- Replies: 2
- Views: 158
Re: How do you oxidize a formula?
Oxidation is just another word for combustion (burning) since combustion involves a substance reacting with oxygen to form carbon dioxide and water vapor. To solve part b of L.7, use dimensional analysis like you did for part a. However, instead of for every 2 mol of fat, there are 110 mol of H2O, p...
- Wed Oct 02, 2019 3:26 pm
- Forum: Molarity, Solutions, Dilutions
- Topic: Calculating molarity
- Replies: 2
- Views: 122
Re: Calculating molarity
You probably could if you wanted to, but it depends on the problem. Mathematically, using mmol/mL is correct, but if the problem asks for the number of moles after giving you the molarity and volume in mL, don't forget to convert to moles instead of mmol. In terms of grading, this would probably be ...
- Wed Oct 02, 2019 3:20 pm
- Forum: Accuracy, Precision, Mole, Other Definitions
- Topic: Moles
- Replies: 5
- Views: 291
Re: Moles
You can use dimensional analysis on this problem. If you are starting with 25.2 kg of UF6, you can convert to g by multiplying by the conversion: 1000 g/1 kg. From there you can use the molar mass of UF6 to convert to moles by multiplying by the conversion: 1 mol UF6/352.0194 g. Then, in a molecule ...
- Wed Oct 02, 2019 3:17 pm
- Forum: Molarity, Solutions, Dilutions
- Topic: General Rounding Question
- Replies: 9
- Views: 353
Re: General Rounding Question
I asked Professor Lavelle this question after the first class. He told me that we should use the full value on whatever periodic table we are given, so if he gives us a periodic table that goes out 3 decimal places, write the entire decimal. I believe this is just to prevent rounding errors since we...
- Wed Oct 02, 2019 3:11 pm
- Forum: Accuracy, Precision, Mole, Other Definitions
- Topic: Homework Problem E.15
- Replies: 4
- Views: 204
Re: Homework Problem E.15
Essentially, what Thomas and Jillian explained is correct. However, if you are still confused, another way of thinking about this question is to put it in the format of an equation. You know that (mass of mystery metal) + (mass of (OH)2) = 74.10 g/mol. Using the masses of oxygen and hydrogen from yo...

