Search found 157 matches
- Sat Mar 14, 2020 1:37 pm
- Forum: Administrative Questions and Class Announcements
- Topic: ENDGAME Review Session
- Replies: 71
- Views: 5703
Re: ENDGAME Review Session
For 1E, I thought increasing the concentration of Ag+ on the cathode side would increase the cell potential. In the Nernst equation for a concentration cell where Q=[anode]/[cathode], increasing the cathode concentration will decrease Q which makes the term more negative which means a more positive ...
- Sat Mar 14, 2020 1:32 pm
- Forum: Arrhenius Equation, Activation Energies, Catalysts
- Topic: A- frequency factor
- Replies: 2
- Views: 291
Re: A- frequency factor
The frequency factor tells us about the reaction mechanism and angles in which the reactants collide. It kind of indicates the ratio of collisions that occur at the correct angle to form products. This is useful in the Arrhenius equation lnk=lnA-\frac{E_{A}}{RT} when you want to find the activation ...
- Sat Mar 14, 2020 1:28 pm
- Forum: Arrhenius Equation, Activation Energies, Catalysts
- Topic: arrhenius eqn.
- Replies: 3
- Views: 273
Re: arrhenius eqn.
I assume you are referring to the derivation of the Arrhenius equation in which ln\frac{k2}{k1}=\frac{A_{E}}{R}[\frac{1}{T_{1}}-\frac{1}{T_{2}}] . It doesn't matter what T1 or T2 are as long as they correspond to the correct k1 and k2. So if you want to find what the rate constant is at a higher tem...
- Wed Mar 11, 2020 9:32 am
- Forum: Administrative Questions and Class Announcements
- Topic: Test 2 Grades
- Replies: 22
- Views: 1272
Re: Test 2 Grades
My TA emailed us and said she could hand them back sometime Friday in person for anyone who would want it. I would wait to see if your TA says anything about it.
- Wed Mar 11, 2020 9:29 am
- Forum: Kinetics vs. Thermodynamics Controlling a Reaction
- Topic: Outline 6 Learning Objective
- Replies: 3
- Views: 398
Re: Outline 6 Learning Objective
I think this has to do with the unique rate and the differential rate law. Remember that the rate of change of reactants and products relative to each other depends on their stoichiometric coefficients. For example in the reaction 2A\rightarrow B , the reactant A is used up twice as fast as the prod...
- Wed Mar 11, 2020 9:23 am
- Forum: Concepts & Calculations Using Second Law of Thermodynamics
- Topic: Units for Thermo Calculations
- Replies: 3
- Views: 411
Re: Units for Thermo Calculations
It doesn't really matter if you use kJ or J BUT you must use the same units in your equation. For example, if  is given in kJ/mol but
is given in kJ/mol but 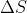 is given in J mol-1 K-1, you HAVE to convert either kJ to J or vice versa to have consistent units.
is given in J mol-1 K-1, you HAVE to convert either kJ to J or vice versa to have consistent units.
- Wed Mar 11, 2020 9:20 am
- Forum: Second Order Reactions
- Topic: Finding out order
- Replies: 22
- Views: 1058
Re: Finding out order
Just to expand on the previous comment, you can generalize the rate constant's units as \frac{1}{M^{n-1}s} where n is the order of the reaction. So for a zero order reaction, the units would be M/s. For first order, it's just s^-1. For second order, it's \frac{1}{Ms} , etc. The rate constant's units...
- Wed Mar 11, 2020 9:10 am
- Forum: Administrative Questions and Class Announcements
- Topic: ENDGAME Review Session
- Replies: 71
- Views: 5703
Re: ENDGAME Review Session
Thank you Lyndon for all of the work, time, and dedication you have done in helping all of us learn, succeed, and grow in these past two quarters. You have been an absolutely wonderful, smart, and fun UA and your review sessions have helped all of us improve our understanding and skills. I know you ...
- Thu Mar 05, 2020 12:13 pm
- Forum: General Rate Laws
- Topic: finding order of reactant with three reactants
- Replies: 1
- Views: 230
Re: finding order of reactant with three reactants
There actually is a set where the concentration of B changes but A and C don't. Look at experiments 1 and 3. For this, you would divide the final over initial concentration of B and divide the final over initial reaction rates. You should find that B changes by a factor of about 2.4 while the rate c...
- Thu Mar 05, 2020 12:09 pm
- Forum: Work, Gibbs Free Energy, Cell (Redox) Potentials
- Topic: Combustion of gas
- Replies: 6
- Views: 548
Re: Combustion of gas
Under standard conditions, the combustion of gas is most likely spontaneous since you would usually be releasing heat (-delta H) and increasing entropy (+delta S). However, I wouldn't say that combustion is always spontaneous, especially depending on non-standard conditions. For example, if you have...
- Thu Mar 05, 2020 12:06 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Standard potential at cathode and anode
- Replies: 1
- Views: 189
Re: Standard potential at cathode and anode
For a galvanic cell to work and provide a spontaneous current, you do NOT need both (or any) half-reactions to have a positive standard reduction potential. Remember that for these problems you want to find the E cell of the OVERALL reaction. If the cathode has a higher reduction potential than the ...
- Thu Mar 05, 2020 12:02 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: Problem 6.63
- Replies: 2
- Views: 271
Re: Problem 6.63
When working with a pH electrode meter you have the following equation: E=E^{\circ}-\frac{RT}{F}ln[H^{+}] . This is derived from the Nernst equation where in this case, Q is just the concentration of H+ because in buffers, [HA] and [A-] are the same. From here, you know that you are given the cell p...
- Thu Mar 05, 2020 11:56 am
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Delta G and Delta G naught
- Replies: 2
- Views: 246
Re: Delta G and Delta G naught
\Delta G^{\circ} refers to the change in Gibbs free energy of a reaction under STANDARD conditions (298K and 1 atm/barr or 1M). \Delta G is unique to a reaction under certain conditions that are NOT STANDARD like a different temperature or concentrations. The equation of \Delta G=\Delta G^{\circ}+R...
- Thu Mar 05, 2020 11:50 am
- Forum: Balancing Redox Reactions
- Topic: Question on 6L.7
- Replies: 2
- Views: 258
Re: Question on 6L.7
To study the reaction given, you have to use both oxidation and reduction half-reactions to end up with AgBr (s) from Ag+ and Br-. If your Ag+ is being reduced to form Ag (s), you know that there must be another oxidation half-reaction that uses Br- to form your desired AgBr(s) (not just Br). If you...
- Thu Mar 05, 2020 11:45 am
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: Hw 6N.3 a)
- Replies: 2
- Views: 295
Re: Hw 6N.3 a)
So from the cell diagram, we know that the anode on the left is the oxidation half-reaction and the cathode on the right is the reduction half-reaction. For the oxidation half-reaction we have two species they give you, H 2 (g) and HCl (aq). In this case, since HCl is aqueous, you can think of it as...
- Wed Mar 04, 2020 4:04 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: 6M.5
- Replies: 1
- Views: 193
Re: 6M.5
Remember that you can only use one method to find the E cell . If you use the equation E^{\circ}_{cell}=E^{\circ}_{cathode}-E^{\circ}_{anode} , you do NOT flip any signs and only use the reduction potentials as given in the chart. In your example, you would find the reduction potential of NO 3 - (0....
- Wed Mar 04, 2020 3:48 pm
- Forum: Reaction Mechanisms, Reaction Profiles
- Topic: Rate-Determining Step
- Replies: 4
- Views: 414
Re: Rate-Determining Step
The rate-determining step (aka the slow step) can be identified using the rate law given. For example, in class we were given a rate law of Rate = k [NO 2 ] 2 . In this case, we would know that the rate-determining step is the one that leads to that rate law, which in this case is the bimolecular co...
- Thu Feb 27, 2020 4:48 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: 6L7 (a) HW
- Replies: 1
- Views: 175
Re: 6L7 (a) HW
In this case, the given reaction is the overall cell reaction and doesn't seem to have any change in oxidation numbers for reactants and products. However, the oxidation and reduction half-reactions will. This might not be the best way to determine the half-reactions, but I honestly just looked at t...
- Thu Feb 27, 2020 4:36 pm
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: log vs. ln in Nernst
- Replies: 2
- Views: 266
Re: log vs. ln in Nernst
I think what he was trying to say was that when plotted, the ln or log of a variable will appear linear when graphed, but in reality, the values are exponentially related. So even though the value for the logQ may be only 1 higher, the actual value for Q will be 10 times as much. If log is 2 higher,...
- Thu Feb 27, 2020 4:27 pm
- Forum: Work, Gibbs Free Energy, Cell (Redox) Potentials
- Topic: Homework 6M.5
- Replies: 2
- Views: 219
Re: Homework 6M.5
Hmm, I'm not sure but the solutions manual includes NO not O. The cell diagram you should get is Hg(l)|Hg22+(aq)||NO3-(aq), H+(aq)|NO(g)|Pt(s). This should make sense since the anode is where Hg is oxidized into Hg22+ and the N in NO3- is reduced from +5 to +2 in NO.
- Thu Feb 27, 2020 4:18 pm
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: 6N3A
- Replies: 4
- Views: 398
Re: 6N3A
Why is E^{\circ} = 0? In what scenarios is E^{\circ} = 0? In this problem, there are actually two reasons why E^{\circ} is 0. Remember that the standard cell potential for a galvanic cell is equal to the standard reduction potential of the cathode minus the standard reduction potential of the anode...
- Wed Feb 26, 2020 8:10 pm
- Forum: Appications of the Nernst Equation (e.g., Concentration Cells, Non-Standard Cell Potentials, Calculating Equilibrium Constants and pH)
- Topic: 6N3A
- Replies: 4
- Views: 398
Re: 6N3A
n should be the moles of electrons that are transferred in the redox reaction. In the problem, you are given the cell diagram and can figure out the oxidation (anode) and reduction (cathode) reactions and how many electrons are involved in each (should be the same when balanced). For part a, you hav...
- Wed Feb 26, 2020 7:58 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: 6M.5
- Replies: 1
- Views: 195
Re: 6M.5
The reason why the left side (anode) doesn't have the platinum electrode is because Hg (l) is a special case in that it can also act as a conductor. In this case, a wire could be inserted into the mercury which would be able to become oxidized, losing two electrons and sending a current through the ...
- Wed Feb 19, 2020 8:14 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Midterm 3B
- Replies: 2
- Views: 840
Re: Midterm 3B
You can refer to the equation q=mC\Delta T to think about this problem. From this equation, we know that a greater q means a greater change in temperature and that a larger mass means a lower change in temperature. As the moles of HCl and NaOH react, the formation of water releases energy as heat (q...
- Wed Feb 19, 2020 8:10 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: Gibbs Free Energy Equation that relates K
- Replies: 4
- Views: 356
Re: Gibbs Free Energy Equation that relates K
\Delta G^{\circ} refers to the Gibbs free energy of a reaction at the STANDARD state whereas \Delta G refers to the Gibbs free energy under other non-standard conditions. For example, you might want to find \Delta G when the reaction is occurring at a different temperature T or when it's not at equ...
- Wed Feb 19, 2020 8:07 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Midterm Q3B and Q3D
- Replies: 1
- Views: 169
Re: Midterm Q3B and Q3D
3B: You can refer to the equation q=mC\Delta T to think about this problem. From this equation, we know that a greater q means a greater change in temperature and that a larger mass means a lower change in temperature. As the moles of HCl and NaOH react, the formation of water releases energy as hea...
- Wed Feb 19, 2020 7:58 pm
- Forum: Galvanic/Voltaic Cells, Calculating Standard Cell Potentials, Cell Diagrams
- Topic: i
- Replies: 1
- Views: 146
Re: i
I believe the i represents current. In a galvanic cell, you have the maximum potential difference as the current approaches 0. This should make sense since if you have more current, then the difference in potentials will decrease at a faster rate since the charges are slowly becoming neutralized (he...
- Wed Feb 19, 2020 7:51 pm
- Forum: Balancing Redox Reactions
- Topic: balancing redox reactions where the oxidizing agent and reducing agent are the same
- Replies: 2
- Views: 786
Re: balancing redox reactions where the oxidizing agent and reducing agent are the same
In these cases, you would have two separate half-reactions with the same reactant for both the oxidation half-reaction and reduction half-reaction. In the case of Br2 that you gave, the oxidation half-reaction would be Br_{2} (l)+12OH^{-}(aq)\rightarrow 2BrO_{3}^{-}(aq)+6H_{2...
- Wed Feb 19, 2020 7:35 pm
- Forum: Gibbs Free Energy Concepts and Calculations
- Topic: 5G 21
- Replies: 1
- Views: 186
Re: 5G 21
In this problem, you are solving for the change in gibbs free energy of the reaction by adding/subtracting standard gibbs free energy of formation of the products and reactants. The equation is \Delta G_{rxn}^{\circ} = \sum \Delta G^{\circ}_{f}(products)-\sum \Delta G^{\circ}_{f}(reactan...
- Tue Feb 11, 2020 3:58 pm
- Forum: Entropy Changes Due to Changes in Volume and Temperature
- Topic: How to think of entropy?
- Replies: 2
- Views: 114
Re: How to think of entropy?
Entropy can be thought of as the possible number of positions or orientations a molecule can occupy (these are called microstates). Basically, if there is more space to occupy, more possible configurations, more thermal movement, etc. then there is more entropy. (Honestly you can still think of it a...
- Tue Feb 11, 2020 3:56 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Heat Capacity for Enthalpy
- Replies: 2
- Views: 204
Re: Heat Capacity for Enthalpy
If you are using moles (n), then C will be the molar heat capacity. Make sure that in problems, if you're only given the molar heat capacity, that you change any mass into moles. Specific heat capacity will only work for mass (g).
- Tue Feb 11, 2020 3:55 pm
- Forum: Calculating Standard Reaction Entropies (e.g. , Using Standard Molar Entropies)
- Topic: State of matter affect entropy
- Replies: 3
- Views: 270
Re: State of matter affect entropy
The standard entropy of a reaction will generally increase (+delta S) if you go from solid to liquid/gas or liquid to gas because you are going from more ordered, confined molecules to a lot more space and more possible states for the molecules. There's more space and movement in a gas than a solid ...
- Tue Feb 11, 2020 3:53 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: equipartition theorum
- Replies: 2
- Views: 257
Re: equipartition theorum
Yep! On the equation sheet on the cover of the test there should be the equations for the molar heat capacities of monatomic ideal gases at constant pressure and constant volume. I believe that Cp = (5/2)R and Cv = (3/2)R.
- Tue Feb 11, 2020 3:52 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Heat capacity
- Replies: 3
- Views: 203
Re: Heat capacity
Typically, if you are given a mass of molecules in a reaction, you will use the specific heat capacity because q=mC_{s}\Delta T where m is mass (in grams) and C is the specific heat. However, if you are only given the molar heat capacity you will need to convert the mass to moles so that it satisfie...
- Tue Feb 11, 2020 3:49 pm
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Adding Inert Gas
- Replies: 20
- Views: 1134
Re: Adding Inert Gas
Adding an inert gas will increase the pressure of a system. However, it does NOT affect the equilibrium of a reaction because there is no change in concentration which means Q does not change. Adding an inert gas will not shift a reaction towards products or reactants.
- Tue Feb 11, 2020 3:45 pm
- Forum: Concepts & Calculations Using First Law of Thermodynamics
- Topic: Meaning of q=-w
- Replies: 14
- Views: 2365
Re: Meaning of q=-w
Knowing that q=-w when the change in internal energy is 0 just helps you solve problems that may require work (but you're only given heat) or vice versa. You know that the work done by a system expanding isothermally is the opposite sign of the heat coming into the system. Note that the internal ene...
- Wed Feb 05, 2020 9:06 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Cp and Cv
- Replies: 10
- Views: 483
Re: Cp and Cv
For ideal gases, you might want to know that for an atom Cp=(5/2)R and Cv=(3/2)R. I believe the first one is given on the equation sheet.
- Wed Feb 05, 2020 8:58 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Standard State of Compounds
- Replies: 2
- Views: 156
Re: Standard State of Compounds
I think what your TA meant is that substances in their standard state in their most stable form have a standard enthalpy of formation of 0. The most stable form of an element is one that only contains that element in its natural state. For example, oxygen's most stable form is O2 gas so the enthalpy...
- Wed Feb 05, 2020 8:48 pm
- Forum: Third Law of Thermodynamics (For a Unique Ground State (W=1): S -> 0 as T -> 0) and Calculations Using Boltzmann Equation for Entropy
- Topic: Boltzmann Equation Notes
- Replies: 2
- Views: 160
Re: Boltzmann Equation Notes
All this means is that in the Boltzmann Equation S=k_{B}lnW , if you have a large error in W (degeneracy), you're not going to have much of an error in S (entropy). So you might have a large difference in values you get for W, but once you take the natural log and multiply by the Boltzmann constant,...
- Wed Feb 05, 2020 8:34 pm
- Forum: Concepts & Calculations Using Second Law of Thermodynamics
- Topic: Entropy as three steps
- Replies: 2
- Views: 167
Re: Entropy as three steps
I also had a question on this problem. I get how to do the problem using the entropy equations when temperature changes. But why is it that you can cool the water vapor back down to 85 Centigrade and still have it be in the vapor state? I assume it's in the vapor state since the molar heat capacity ...
- Tue Feb 04, 2020 4:57 pm
- Forum: Concepts & Calculations Using Second Law of Thermodynamics
- Topic: Temperature in Second Law
- Replies: 2
- Views: 110
Re: Temperature in Second Law
I believe you need to convert the temperature to Kelvin. This is because the units for entropy are Joules per Kelvin. So 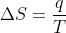 will only give you the right units for entropy if you use Kelvin not Celsius.
will only give you the right units for entropy if you use Kelvin not Celsius.
- Tue Feb 04, 2020 4:51 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: 4D15
- Replies: 1
- Views: 83
Re: 4D15
So for this problem you're actually given the enthalpies of combustion not the enthalpies of formation (the 3rd method we discussed in class). Basically what the enthalpy of combustion means is the amount of heat given off/absorbed when a mole of that molecule reacts in a combustion reaction. For ex...
- Tue Feb 04, 2020 4:47 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: adding enthalpy
- Replies: 1
- Views: 90
Re: adding enthalpy
In this problem, you need to find equations for both the heat change of the ice and the water to find the final temperature. The heat change (q) of the ice involves both the enthalpy of fusion (what you're confused about) and the heat needed to increase the temperature of the melted ice. \Delta H_{f...
- Mon Feb 03, 2020 10:05 pm
- Forum: Reaction Enthalpies (e.g., Using Hess’s Law, Bond Enthalpies, Standard Enthalpies of Formation)
- Topic: Problem 4C.3a
- Replies: 1
- Views: 80
Re: Problem 4C.3a
Yep! The change in enthalpy is +765J since 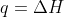 under constant pressure.
under constant pressure.
- Thu Jan 30, 2020 9:04 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: Work of expansion equation
- Replies: 4
- Views: 170
Re: Work of expansion equation
Just as an extra note: If the system were to be compressed, work would be done on the system by the surroundings. In this case, the sign for work would be positive since it is gaining energy from the surroundings. You'd still use the same equation of w=-P\Delta V ; it's just that if compressed, delt...
- Thu Jan 30, 2020 9:01 pm
- Forum: Heat Capacities, Calorimeters & Calorimetry Calculations
- Topic: When do we need to consider the calorimeter?
- Replies: 2
- Views: 159
Re: When do we need to consider the calorimeter?
Sometimes a calorimeter can be considered a surrounding but usually the problem will make that clear. For example, if you look at problem 4D.3, the calorimeter absorbs heat as a result of the exothermic reaction between gases. In that case, you would consider q (heat) as the transfer in energy betwe...
- Thu Jan 30, 2020 8:35 pm
- Forum: Thermodynamic Systems (Open, Closed, Isolated)
- Topic: Isolated// Energy
- Replies: 11
- Views: 609
Re: Isolated// Energy
An isolated system has no transfer of matter or energy.
From the equation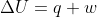 , it should make sense that the change in internal energy of an isolated system will be 0 because there is no heat transfer (energy) and no work being done (also energy).
, it should make sense that the change in internal energy of an isolated system will be 0 because there is no heat transfer (energy) and no work being done (also energy).
From the equation
- Wed Jan 29, 2020 10:26 pm
- Forum: Thermodynamic Systems (Open, Closed, Isolated)
- Topic: Define Reversible process
- Replies: 2
- Views: 253
Re: Define Reversible process
A reversible expansion is basically one that takes place in really small, slow steps. Conversely, an irreversible expansion is one that happens very quickly. I'll give examples of both. So for both processes, you can think of a beaker in which a piston is compressing a gas at say 2 atm. In an irreve...
- Wed Jan 29, 2020 10:19 pm
- Forum: Calculating Work of Expansion
- Topic: 4A.5
- Replies: 3
- Views: 108
Re: 4A.5
Here's a link to a rough visual I created: https://ibb.co/XC7dP1d
Hopefully it can clarify the last part of my post :)
Hopefully it can clarify the last part of my post :)
- Wed Jan 29, 2020 9:43 pm
- Forum: Calculating Work of Expansion
- Topic: 4A.5
- Replies: 3
- Views: 108
Re: 4A.5
To answer your first question: in this case, the system that does more work will have the more negative work (or a greater absolute value). Remember that our equation for work resembles w=-P\Delta V . If the volume changes more, then more work is done and the value is more negative. We make the sign...
- Wed Jan 22, 2020 9:10 pm
- Forum: Phase Changes & Related Calculations
- Topic: Enthalpy
- Replies: 4
- Views: 357
Re: Enthalpy
Enthalpy doesn't necessarily mean a change in temperature; it's just a measure of the heat energy being released/absorbed. For example, when water is undergoing a phase change to become vapor, there is a positive \Delta H value because heat is being applied for vaporization BUT the temperature will ...
- Wed Jan 22, 2020 3:41 pm
- Forum: Administrative Questions and Class Announcements
- Topic: 14A final solutions
- Replies: 6
- Views: 269
Re: 14A final solutions
I doubt the solutions would be posted anywhere. I don't believe they're on the chem 14a website.
- Wed Jan 22, 2020 3:37 pm
- Forum: Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions
- Topic: Buffers
- Replies: 3
- Views: 218
Re: Buffers
I believe Lavelle was talking about how one can combine a weak acid and a salt solution containing its conjugate base to make a buffer. (The same applies with a weak base and its salt with the conjugate acid). A buffer is a solution that can resist changes in pH and balance the pH if an acid or base...
- Wed Jan 22, 2020 3:25 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: 5I.27
- Replies: 1
- Views: 119
Re: 5I.27
Yeah, I think this is just an error in the solutions manual. The values you put in the quadratic equation are correct based on the ICE table and Kc. The solutions manual says that the values for x are 9.2 and .071 BUT you should've gotten 8.7 and .074. In this way, solving for the equilibrium concen...
- Wed Jan 22, 2020 9:49 am
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: ice box approximation
- Replies: 9
- Views: 345
Re: ice box approximation
Again, in addition to the small (<10 -3 ) approximation, you can find the percent ionization to test if you can use the approximation. If the percent ionization is less than 5%, your approximation is acceptable. Remember that to find percent ionization, you take the equilibrium concentration of the ...
- Wed Jan 22, 2020 9:44 am
- Forum: Acidity & Basicity Constants and The Conjugate Seesaw
- Topic: 6D.15 part b
- Replies: 3
- Views: 274
Re: 6D.15 part b
If you took Lavelle for 14A, we did cover the transition metals acting as acids in salts briefly. Remember that transition metals like aluminum can form coordination compounds with ligands including water when in solution. There are 6 available bonding sites in an octahedral arrangement where the al...
- Tue Jan 14, 2020 9:18 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Acids and Bases
- Replies: 6
- Views: 254
Re: Acids and Bases
Like others have said, acid and base reactions are not always at equilibrium. However, it is important to note that when we do calculations for pH, pK a , and pK b , these values will be based on equilibrium concentrations. This is especially important when considering weak acids/bases' dissociation...
- Tue Jan 14, 2020 2:36 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Strong Acids/Bases
- Replies: 4
- Views: 157
Re: Strong Acids/Bases
To add on to previous comments, since weak bases do not fully dissociate into their respective ions, you will have concentrations of both reactant and product at equilibrium. This is the basis for K a which is equal to the concentration of the dissociated products over the remaining concentration of...
- Mon Jan 13, 2020 9:16 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Homework 5.39
- Replies: 1
- Views: 85
Re: Homework 5.39
I used the information in table 5G.2 to help figure the problem out. If you look at that table, you'll find the equilibrium constant for the reverse reaction ( N_{2}O_{4} (g) \rightleftharpoons 2NO_{2} (g) ) at 298 K. From here you should be able to figure out K for the given reactio...
- Mon Jan 13, 2020 7:51 pm
- Forum: Calculating pH or pOH for Strong & Weak Acids & Bases
- Topic: pH range
- Replies: 4
- Views: 287
Re: pH range
Yes, it is indeed possible to have pH outside of the range from 0 to 14. For example, if you had a solution of 10M HCl (a strong acid), you would find the pH = -log(H+) to be -1. Of course, this would be a tremendously strong acid solution. Likewise for very strong bases, if you had a solution of 10...
- Mon Jan 13, 2020 7:47 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: 6A.21
- Replies: 1
- Views: 100
Re: 6A.21
To do this problem, you would use the same concept that we used when finding the concentration of H 3 O + and OH - at the normal room temperature of 25C. Remember that if the solution is neutral, that means that both concentrations of H 3 O + and OH - are equal. The problem gives us that K w is 2.1 ...
- Wed Jan 08, 2020 4:12 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Kc and Kp
- Replies: 8
- Views: 316
Re: Kc and Kp
when a question simply asks for "the equilibrium constant" or "K" does that mean related to concentration or pressure? and why is this so easily interchangeable? I believe that when a problem asks you to find the equilibrium constant, unless it specifies to find Kc or Kp, you sh...
- Wed Jan 08, 2020 4:08 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Are Both L and Aq Excluded From Equilibrium Constant Expressions?
- Replies: 4
- Views: 289
Re: Are Both L and Aq Excluded From Equilibrium Constant Expressions?
So, I know that solids and liquids are not supposed to be included in Kc but I was wondering if Aq is excluded as well. Isn’t Aq liquid too? Also, I still don’t really understand why these things are excluded from the expression so can someone please explain to me why. Aq is included in the equilib...
- Wed Jan 08, 2020 2:47 pm
- Forum: Non-Equilibrium Conditions & The Reaction Quotient
- Topic: Clarification on Q<K and R&P concentrations
- Replies: 2
- Views: 119
Clarification on Q<K and R&P concentrations
In lecture, Dr. Lavelle had on the slides that if Q<K, then [R]>[P] and the forward reaction is favored. I understand that when Q is less than K that means the ratio of products over reactants is less, signifying that there are more reactants (and less products) at that time than there would be at e...
- Wed Jan 08, 2020 2:39 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Textbook question 5I.11
- Replies: 1
- Views: 98
Re: Textbook question 5I.11
Could someone please explain how to get part a? I keep getting 13.9 instead of 6.9 Alright I think I know where you went wrong. When you're finding the reaction quotient Q c for an equilibrium reaction with concentration you MUST make sure that you are using the correct concentrations. In the probl...
- Mon Jan 06, 2020 9:04 pm
- Forum: Equilibrium Constants & Calculating Concentrations
- Topic: Heterogeneous Equilibria
- Replies: 2
- Views: 249
Re: Heterogeneous Equilibria
For heterogeneous equilibria, you will use the same general equation to find the equilibrium constant as for homogeneous equilibria: K_{c}=\frac{[C]^c[D]^d}{[A]^a[B]^b} . However, in heterogeneous equilibria, you will NOT include any solids or pure liquids in the formula. I believe this is because t...
- Mon Jan 06, 2020 8:53 pm
- Forum: Ideal Gases
- Topic: Dynamic Equilibrium
- Replies: 10
- Views: 411
Re: Dynamic Equilibrium
I believe what is meant by dynamic equilibrium is that when a reaction is in equilibrium, there still are reactions occurring. It is not a static system. When a reaction is at equilibrium, concentrations of reactant and product are the same because the reactants are forming products at the same rate...
- Sat Dec 07, 2019 8:06 pm
- Forum: *Molecular Orbital Theory (Bond Order, Diamagnetism, Paramagnetism)
- Topic: Bond order
- Replies: 2
- Views: 2158
Re: Bond order
I think bond order just means is it single, double, or triple bond. So the bond order for Cl2 is 1 since it's a single bond.
- Sat Dec 07, 2019 7:59 pm
- Forum: Calculating pH or pOH for Strong & Weak Acids & Bases
- Topic: ph concept
- Replies: 5
- Views: 353
Re: ph concept
please quote my question so i get a notification, thanks in advance! i know there is something wrong with my thought process, but i cannot figure it out. my thought process is that if a base is weaker, less oh- will be dissociated out. taking the -log of that smaller number will result in a smaller...
- Sat Dec 07, 2019 7:36 pm
- Forum: Properties & Structures of Inorganic & Organic Acids
- Topic: Oxoacid Strength
- Replies: 6
- Views: 736
Re: Oxoacid Strength
Yep, HOF is the stronger acid! When the acids dissociate and donate their proton, you will be left with the anions, FO - and ClO - . Since F is more electronegative it will be able to better delocalize the negative charge of the Oxygen by pulling the electrons closer to it. This delocalization/induc...
- Sat Dec 07, 2019 7:12 pm
- Forum: Photoelectric Effect
- Topic: Dino Nuggets question 8b
- Replies: 3
- Views: 514
Re: Dino Nuggets question 8b
For reference: 8b) A newly designed laser pointer with a certain frequency is pointed at a sodium metal surface. An electron is ejected from the metal surface with wavelength 1.10 nm. What is the frequency of the light from the laser pointer? The work function of sodium is 150.6 kJ∙mol-1. I'll just ...
- Sat Dec 07, 2019 6:55 pm
- Forum: Interionic and Intermolecular Forces (Ion-Ion, Ion-Dipole, Dipole-Dipole, Dipole-Induced Dipole, Dispersion/Induced Dipole-Induced Dipole/London Forces, Hydrogen Bonding)
- Topic: determining number of hydrogen bonding sites
- Replies: 3
- Views: 386
Re: determining number of hydrogen bonding sites
Recall that hydrogen bonds can form when a hydrogen that is bonded to a Nitrogen, Oxygen, or Fluorine is strongly attracted to a Nitrogen, Oxygen, or Fluorine with a lone pair. In problems where it asks you to find how many hydrogen bonding sites there are, you should look for the hydrogens bonded t...
- Wed Dec 04, 2019 3:20 pm
- Forum: Bronsted Acids & Bases
- Topic: HOI vs HOCl
- Replies: 3
- Views: 393
Re: HOI vs HOCl
HOCl and HOI are both oxoacids and act quite differently, because of the oxygen involved in their structure. Since the electrons are withdrawn (or pulled due to the high electronegativity of oxygen and chlorine), they are delocalized and are more prone to get rid of the H+ proton than HOI. But when...
- Wed Dec 04, 2019 3:05 pm
- Forum: Conjugate Acids & Bases
- Topic: Conjugate Acids and Bases (Uses)
- Replies: 1
- Views: 224
Re: Conjugate Acids and Bases (Uses)
I think we should know conjugate acids/bases because they are related to the strength of acids/bases and how salts can or cannot affect pH of a solution. The conjugate base of an acid is basically the product once the acid donates the proton (assuming Bronsted acid). For example, the conjugate base ...
- Wed Dec 04, 2019 2:48 pm
- Forum: Interionic and Intermolecular Forces (Ion-Ion, Ion-Dipole, Dipole-Dipole, Dipole-Induced Dipole, Dispersion/Induced Dipole-Induced Dipole/London Forces, Hydrogen Bonding)
- Topic: intermolecular energy equation
- Replies: 1
- Views: 560
Re: intermolecular energy equation
The interaction potential energy equation says that the energy/strength of an intermolecular force is directly proportional to the polarizability of the two molecules and inversely proportional to the distance between them. You don't need to do any calculations with this but you should be able to co...
- Tue Dec 03, 2019 10:54 am
- Forum: Conjugate Acids & Bases
- Topic: conjugate base and pH
- Replies: 4
- Views: 2064
Re: conjugate base and pH
The conjugate base of a strong acid will not affect the pH because it is weak and stable. For example, if you have a Cl- anion in a salt, that is the conjugate base of HCl, a strong acid. Remember that strong acids will completely dissociate into ions so for HCl: HCl\rightarrow H^{+} + Cl^{-} . Cl- ...
- Tue Dec 03, 2019 10:44 am
- Forum: Identifying Acidic & Basic Salts
- Topic: HW 6D11
- Replies: 7
- Views: 667
Re: HW 6D11
To add onto Benjamin's post: https://slideplayer.com/slide/6819688/23/images/9/Acidity+of+Aqueous+Transition+Metal+Ions.jpg For problem e, the Aluminum cation will form those 6 bonds to water forming a coordination compound. Metal cations will act as acids because the strong positive charge of the m...
- Tue Dec 03, 2019 10:31 am
- Forum: Calculating the pH of Salt Solutions
- Topic: Sig Figs for logarithmic funcitons
- Replies: 6
- Views: 456
Re: Sig Figs for logarithmic funcitons
Actually, the sig figs for pH are a little different than normal. Essentially, if you are calculating the pH from a given concentration, the number of sig figs in the concentration is the number of sig figs you put AFTER the decimal point in the pH. For example, if I was given a 3.14 * 10 -2 M solut...
- Tue Dec 03, 2019 10:24 am
- Forum: Acidity & Basicity Constants and The Conjugate Seesaw
- Topic: Value of Kw?
- Replies: 4
- Views: 624
Re: Value of Kw?
For the calculations we will be doing in 14A, this is the value for K w . All this means is that in water at standard temperature and pressure, the concentration of hydronium ions [H 3 O + ] times the concentration of hydroxide ions [OH - ] is 1 x 10 -14 . This tells us that pH + pOH is 14. However,...
- Tue Dec 03, 2019 10:15 am
- Forum: Acidity & Basicity Constants and The Conjugate Seesaw
- Topic: Confusion about Ph>Pka
- Replies: 2
- Views: 232
Re: Confusion about Ph>Pka
What Dr. Lavelle was talking about was how much an acid will dissociate depending on the acidity of the solution it is in. Remember that for Bronsted acids, the acid in solution can dissociate into hydronium ions (H 3 O + ) and the conjugate base (A - ). For example, HF \rightleftharpoons H^{+} + F^...
- Sat Nov 30, 2019 1:39 pm
- Forum: Calculating pH or pOH for Strong & Weak Acids & Bases
- Topic: Textbook question 6B.3
- Replies: 1
- Views: 110
Re: Textbook question 6B.3
From my understanding, the second solution in part b is diluted relative to the desired solution. Therefore, to find the pH of the diluted acid, you need to find the new concentration/molarity. Using the equation M_{1}V_{1} = M_{2}V_{2} where M1 is .250, V1 is .2000, and V2 is .2500, you can find th...
- Tue Nov 26, 2019 3:59 pm
- Forum: Naming
- Topic: Polydentate
- Replies: 4
- Views: 200
Re: Polydentate
In coordination compounds, a polydentate ligand is a single molecule that can bind to the same central transition metal at multiple sites. This means that it likely will have multiple atoms with lone pairs that can create coordinate covalent bonds with the metal. For this type of question, it's help...
- Tue Nov 26, 2019 3:50 pm
- Forum: Bronsted Acids & Bases
- Topic: Forming a neutralization reaction
- Replies: 2
- Views: 180
Re: Forming a neutralization reaction
Seeing that there are assigned homework problems that are similar to that, I would be prepared to answer those if they do appear on the final.
- Tue Nov 26, 2019 3:49 pm
- Forum: Naming
- Topic: Final Exam
- Replies: 3
- Views: 220
Re: Final Exam
For the final, you should know the ligand names listed in the document Dr. Lavelle has provided: https://lavelle.chem.ucla.edu/wp-content/supporting-files/Chem14A/NamingCoordinationCompounds.pdf I believe the names in the textbook are the new IUPAC ones, but we only need to know how to name coordina...
- Mon Nov 25, 2019 3:46 pm
- Forum: Naming
- Topic: Roman numerals
- Replies: 4
- Views: 247
Re: Roman numerals
The roman numeral indicates the positive charge of the transition metal cation. This notation is often used since transition metals can take on multiple oxidation states. For example, to distinguish Fe 2+ and Fe 3+ you would write Iron (II) or Iron (III). If we have the coordination compound [Co (N...
- Mon Nov 25, 2019 3:35 pm
- Forum: Amphoteric Compounds
- Topic: amphoteric compounds
- Replies: 2
- Views: 162
Re: amphoteric compounds
We know that nonmetal oxides will typically form acidic compounds (ie. H 2 SO 4 or HNO 3 ) and that most metals oxides will form basic compounds (ie. CaO or NaO 2 ). However, amphoteric compounds are ones that can have either acid or base character depending on the reaction. Typically, elements near...
- Mon Nov 25, 2019 3:19 pm
- Forum: Naming
- Topic: Roman numerals
- Replies: 4
- Views: 247
Re: Roman numerals
The roman numeral indicates the positive charge of the transition metal cation. This notation is often used since transition metals can take on multiple oxidation states. For example, to distinguish Fe 2+ and Fe 3+ you would write Iron (II) or Iron (III). If we have the coordination compound [Co (NH...
- Mon Nov 25, 2019 3:12 pm
- Forum: Bronsted Acids & Bases
- Topic: Arrhenius, Bronsted, and Lewis
- Replies: 1
- Views: 174
Re: Arrhenius, Bronsted, and Lewis
The definition of an Arrhenius acid is a molecule that forms a hydronium ion (H 3 O + ) in the presence of water. An Arrhenius base forms a hydroxide ion (OH - ) in water. This definition applies to most Bronsted acids/bases. A Bronsted acid is a molecule that is a proton donor, meaning that it will...
- Tue Nov 19, 2019 10:56 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Instantaneous Dipole Moment
- Replies: 4
- Views: 380
Re: Instantaneous Dipole Moment
I believe this is referring to the fact that at any moment in time, electron distribution in a molecule can be slightly unequal. Electrons may be "hanging out" on one side of a molecule at an instant point in time. This creates a temporary/instantaneous dipole moment because of this unequa...
- Tue Nov 19, 2019 10:45 pm
- Forum: *Molecular Orbital Theory (Bond Order, Diamagnetism, Paramagnetism)
- Topic: SO2 bond sigma and pi bond
- Replies: 4
- Views: 3209
Re: SO2 bond sigma and pi bond
Also, extra stuff not regarding the original question: The VSEPR shape of SO 2 would be bent with a bond angle of slightly less than 120 degrees. This is because there are 3 regions of electron density (trigonal planar derived) but one of them is a lone pair which repels a little more than a bonded ...
- Tue Nov 19, 2019 10:40 pm
- Forum: *Molecular Orbital Theory (Bond Order, Diamagnetism, Paramagnetism)
- Topic: SO2 bond sigma and pi bond
- Replies: 4
- Views: 3209
Re: SO2 bond sigma and pi bond
The Lewis Structure for SO2 is a central Sulfur double bonded to each of the Oxygen atoms. A double bond consists of one sigma bond and one pi bond so in total you would have two sigma bonds and two pi bonds (one sigma and pi for one bonded Oxygen and another sigma and pi for the other).
- Tue Nov 19, 2019 10:35 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Cis vs Trans
- Replies: 1
- Views: 127
Re: Cis vs Trans
I suppose you should know that since there is likely a double bond in the cis and trans isomers, there is a pi bond between p orbitals which prevents the atoms from rotating. This ensures that the cis can't turn into a trans without some kind of chemical reaction.
- Tue Nov 19, 2019 10:32 pm
- Forum: Interionic and Intermolecular Forces (Ion-Ion, Ion-Dipole, Dipole-Dipole, Dipole-Induced Dipole, Dispersion/Induced Dipole-Induced Dipole/London Forces, Hydrogen Bonding)
- Topic: diple induced dipole vs dipole-dipole
- Replies: 1
- Views: 115
Re: diple induced dipole vs dipole-dipole
Dipole-dipole interaction occurs when you have two polar molecules, each with a permanent dipole moment. The negative partial charge of one dipole is attracted to the positive partial charge of the other. For example, if I had H 2 O and NH 3 , the partial positive of the hydrogen would be attracted ...
- Tue Nov 19, 2019 10:26 pm
- Forum: Bond Lengths & Energies
- Topic: Interaction Potential Energy and Radius
- Replies: 2
- Views: 192
Re: Interaction Potential Energy and Radius
When atomic size increases, polarizability ( \alpha ) increases because the electrons are held on less tightly by the nucleus. High polarizability creates the stronger intermolecular forces described by the larger interaction potential energy. The distance between molecules is important but I think ...
- Tue Nov 19, 2019 3:05 pm
- Forum: Interionic and Intermolecular Forces (Ion-Ion, Ion-Dipole, Dipole-Dipole, Dipole-Induced Dipole, Dispersion/Induced Dipole-Induced Dipole/London Forces, Hydrogen Bonding)
- Topic: Hydrogen Bonding
- Replies: 2
- Views: 141
Hydrogen Bonding
I know that hydrogen bonding can occur when a hydrogen that is polar covalently bonded to a N,O, or F has a highly positive partial charge that it is attracted to a lone pair of a different molecule's electronegative atom. Can this other electronegative atom with the lone pair be any atom or does it...
- Mon Nov 18, 2019 5:09 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Structures for test two
- Replies: 1
- Views: 143
Re: Structures for test two
Yes. These have appeared in readings and HW.
You should know linear, trigonal planar, bent (<120 degrees), tetrahedral, trigonal pyramidal, bent (<109.5 degrees), trigonal bipyramidal, seesaw, T-shaped, linear (but with 3 lone pairs), octahedral, square pyramidal, and square planar.
You should know linear, trigonal planar, bent (<120 degrees), tetrahedral, trigonal pyramidal, bent (<109.5 degrees), trigonal bipyramidal, seesaw, T-shaped, linear (but with 3 lone pairs), octahedral, square pyramidal, and square planar.
- Mon Nov 18, 2019 5:06 pm
- Forum: Determining Molecular Shape (VSEPR)
- Topic: Polar/Nonpolar
- Replies: 1
- Views: 251
Re: Polar/Nonpolar
You have to understand the VSEPR shape of the molecule to determine its polarity. AsF 5 has a trigonal bipyramidal shape since it has 5 regions of electron density and no lone pairs. In a trigonal bipyramidal shape, you have 2 axial bonded atoms and 3 equatorial bonded atoms (2 are straight up and d...
- Mon Nov 18, 2019 5:01 pm
- Forum: Interionic and Intermolecular Forces (Ion-Ion, Ion-Dipole, Dipole-Dipole, Dipole-Induced Dipole, Dispersion/Induced Dipole-Induced Dipole/London Forces, Hydrogen Bonding)
- Topic: Strength of IMF
- Replies: 1
- Views: 92
Re: Strength of IMF
I believe that the hydrogen bond (-20 kJ/mol) is indeed stronger than the ion-dipole force (-15 kJ/mol).
- Mon Nov 18, 2019 4:58 pm
- Forum: Ionic & Covalent Bonds
- Topic: Radicals and Bonds
- Replies: 1
- Views: 196
Re: Radicals and Bonds
I believe you would still treat the lone electron as one region of electron density. So it would act similar to a lone pair but have a lower repulsion than a lone pair would.
- Mon Nov 18, 2019 4:54 pm
- Forum: Lewis Structures
- Topic: HW #2.27
- Replies: 1
- Views: 226
Re: HW #2.27
The HCH bond in CH 2 2- has the smallest bond angle because it has a bent molecular shape. You are right in saying that the molecule will be based off a tetrahedral shape since it has 4 regions of electron density (2 bonding pairs and 2 lone pairs). Since it has 2 lone pairs, you would replace 2 ato...

